Question: (? Question 19 (1 point) Retake question At an unspecified temperature, these two gases are combined in a flask. Initially, Q value for the reaction
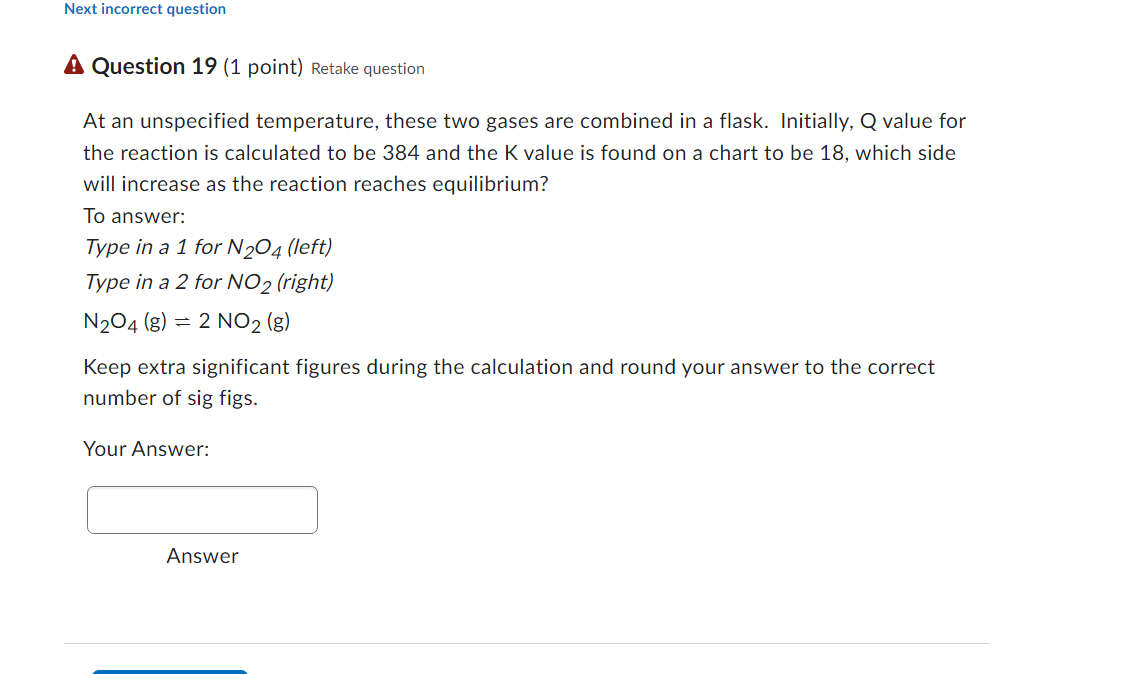
(? Question 19 (1 point) Retake question At an unspecified temperature, these two gases are combined in a flask. Initially, Q value for the reaction is calculated to be 384 and the K value is found on a chart to be 18 , which side will increase as the reaction reaches equilibrium? To answer: Type in a 1 for N2O4 (left) Type in a 2 for NO2 (right) N2O4(g)=2NO2(g) Keep extra significant figures during the calculation and round your answer to the correct number of sig figs. Your
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


