Question: QUESTION 3 a) For the Debye-Hckel limiting law: logy+ = -1272_1A/Tm where A = 210 Namgolv 1/2 1 2.303 3/2 4BT. Show the solution steps
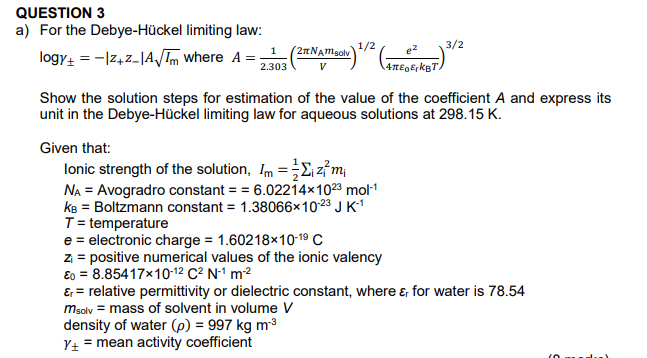
QUESTION 3 a) For the Debye-Hckel limiting law: logy+ = -1272_1A/Tm where A = 210 Namgolv 1/2 1 2.303 3/2 4BT. Show the solution steps for estimation of the value of the coefficient A and express its unit in the Debye-Hckel limiting law for aqueous solutions at 298.15 K. Given that: Ionic strength of the solution, Im = 2 z mi NA = Avogradro constant = = 6.02214x1023 mol-1 ke = Boltzmann constant = 1.38066x10-23 J K1 T = temperature e = electronic charge = 1.60218x10-19 C z = positive numerical values of the ionic valency o = 8.85417x10-12 C2 N1 m2 Er = relative permittivity or dielectric constant, where e for water is 78.54 msolv = mass of solvent in volume V density of water (p) = 997 kg m Y+ = mean activity coefficient
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


