Question: Question 2 0.1 pts Which is the true statement about phase diagram? (a) In phase diagram, there are single phase regions as well as the

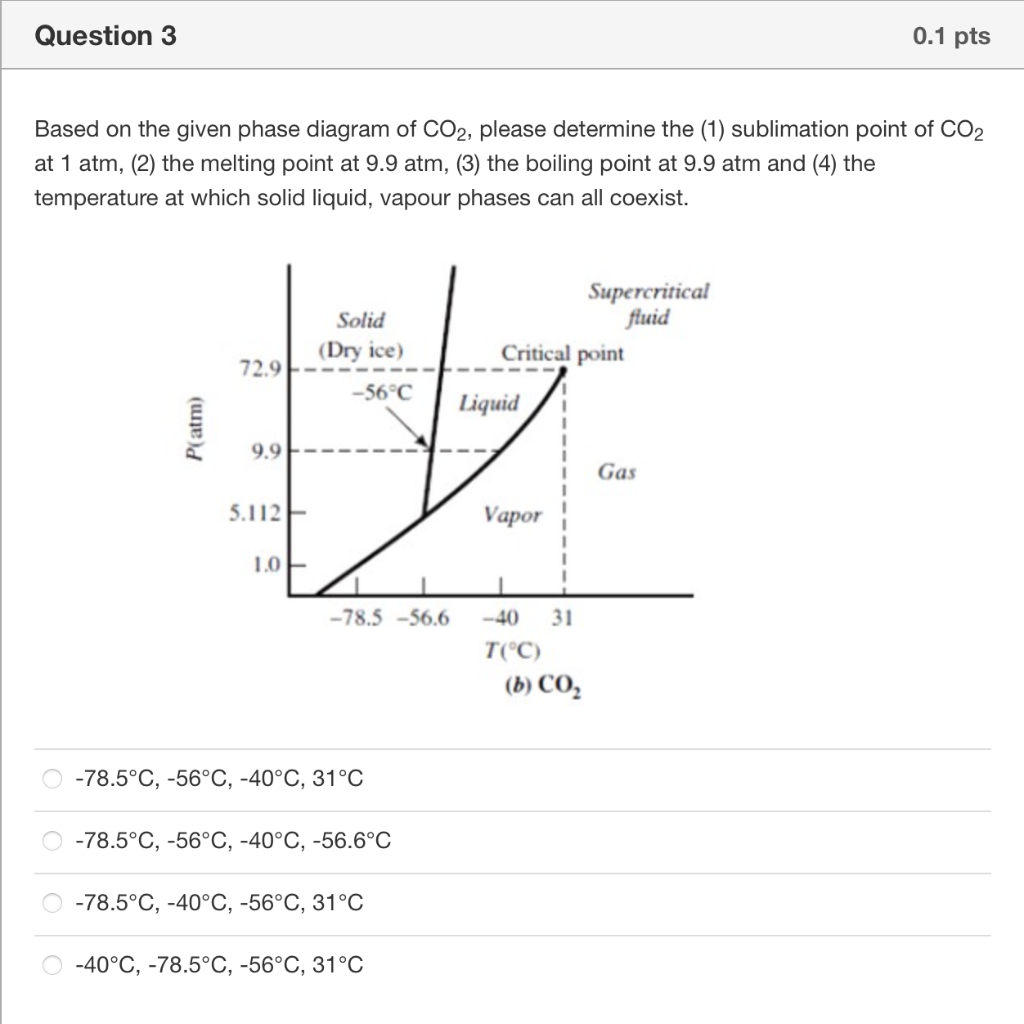
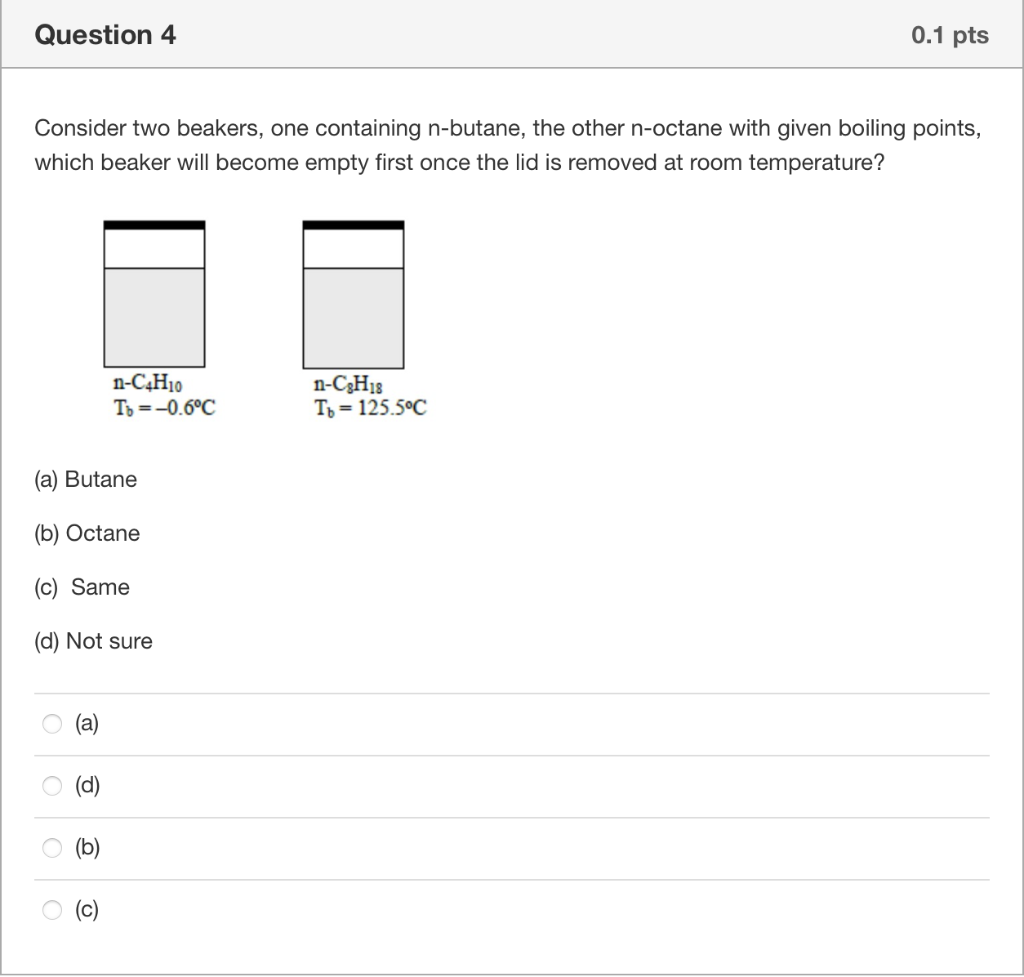
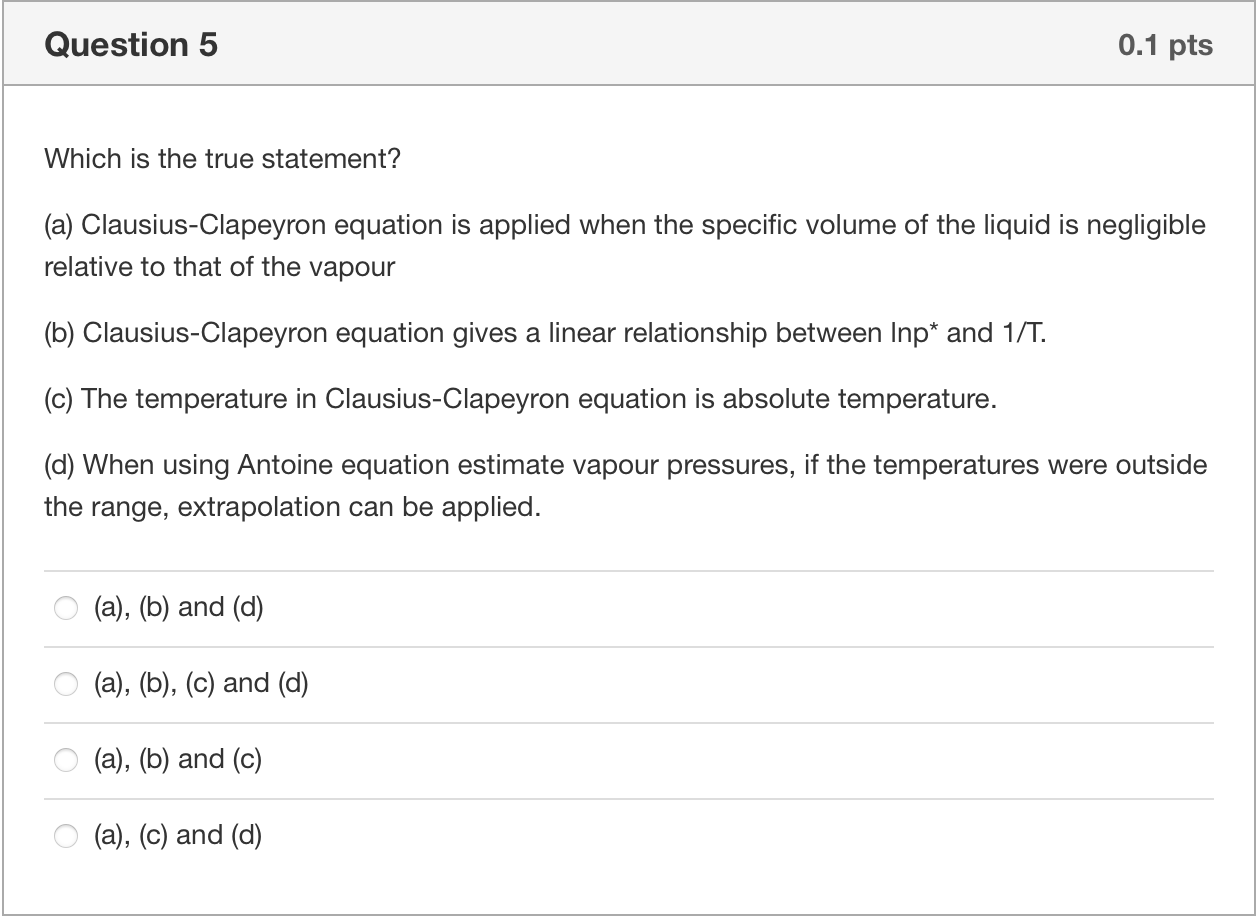
Question 2 0.1 pts Which is the true statement about phase diagram? (a) In phase diagram, there are single phase regions as well as the multiphase coexistence region. (b) Beyond the critical point, a substance can exist as a non-condensable gas. (c) Sublimation point of of species at given pressure can be found at the liquid-solid equilibrium curve. (d) Vapour pressure of the a specie at given temperature can be found at liquid-vapour equilibrium curve or solid-vapour equilibrium curve. (b), (c) and (d) (a), (b), (c) and (d) (a), (b) and (d) (a), (b) and (c) Question 3 0.1 pts Based on the given phase diagram of CO2, please determine the (1) sublimation point of CO2 at 1 atm, (2) the melting point at 9.9 atm, (3) the boiling point at 9.9 atm and (4) the temperature at which solid liquid, vapour phases can all coexist. Supercritical fluid Critical point Solid (Dry ice) -56C 72.9 Liquid P(atm) 9.9 Gas 5.112 Vapor 1.0 -78.5 -56.6 -40 31 T(C) (b) CO, 0 -78.5C, -56C, -40C, 31C -78.5C, -56C, -40C, -56.6C -78.5C, -40C, -56C, 31C 0 -40C, -78.5C, -56C, 31C Question 4 0.1 pts Consider two beakers, one containing n-butane, the other n-octane with given boiling points, which beaker will become empty first once the lid is removed at room temperature? n-C4H10 To = -0.6C n-C3H18 To = 125.5C (a) Butane (b) Octane (c) Same (d) Not sure (a) O (d) (b) b (c) Question 5 0.1 pts Which is the true statement? (a) Clausius-Clapeyron equation is applied when the specific volume of the liquid is negligible relative to that of the vapour (b) Clausius-Clapeyron equation gives a linear relationship between Inp* and 1/T. (c) The temperature in Clausius-Clapeyron equation is absolute temperature. (d) When using Antoine equation estimate vapour pressures, if the temperatures were outside the range, extrapolation can be applied. (a), (b) and (d) (a), (b), (c) and (d) (a), (b) and (c) (a), (c) and (d)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


