Question: Q10a) 8marks Need help with entire question Composition (mol% Al2O3) 20 40 60 80 2800 5000 Liquid 4500 2400 MgO (ss) MgAl 04 () +
Q10a) 8marks Need help with entire question
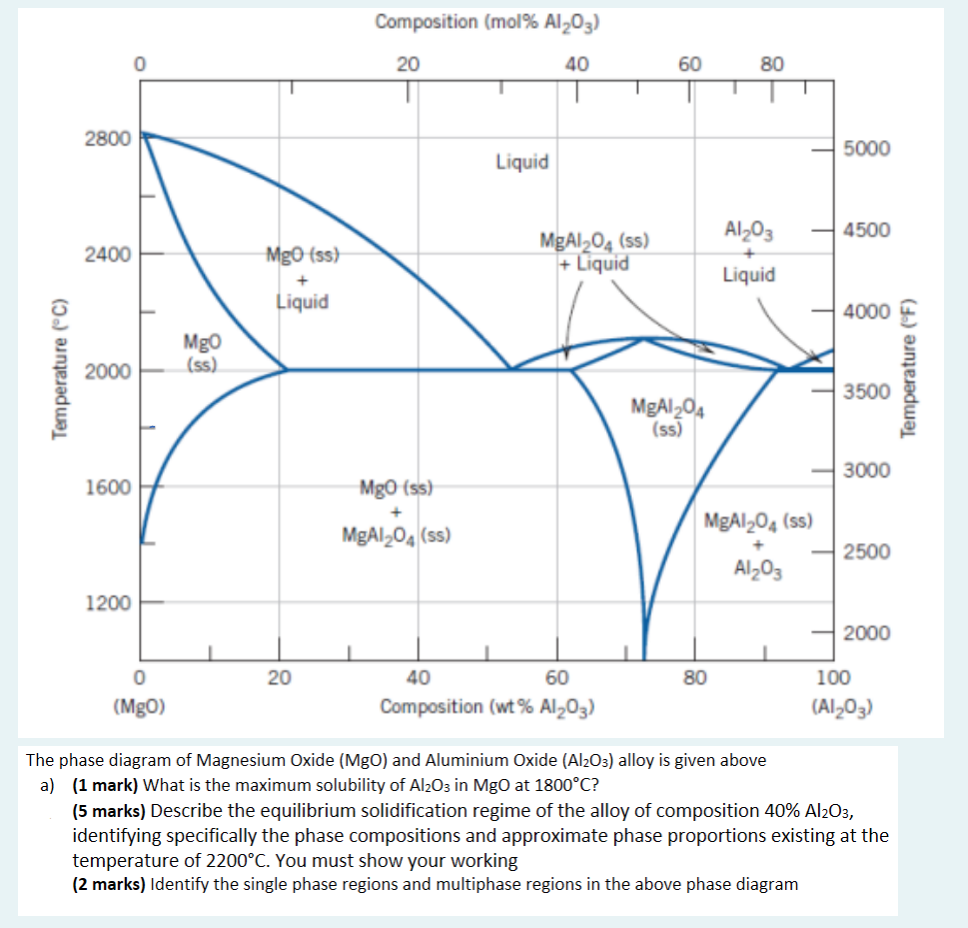
Composition (mol% Al2O3) 20 40 60 80 2800 5000 Liquid 4500 2400 MgO (ss) MgAl 04 () + Liquid Al2O3 Liquid + Liquid 4000 Temperature (C) Mgo (ss) 2000 Temperature (F) 3500 MgA1204 (ss) 3000 1600 Mgo (ss) MgA1204 (ss) MgAI 04 (ss) 2500 Al2O3 1200 2000 20 80 0 (Mgo) 40 60 Composition (wt% Al2O3) 100 (Al2O3) The phase diagram of Magnesium Oxide (MgO) and Aluminium Oxide (Al2O3) alloy is given above a) (1 mark) What is the maximum solubility of Al2O3 in MgO at 1800C? (5 marks) Describe the equilibrium solidification regime of the alloy of composition 40% Al2O3, identifying specifically the phase compositions and approximate phase proportions existing at the temperature of 2200C. You must show your working (2 marks) Identify the single phase regions and multiphase regions in the above phase diagram
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


