Question: Question 2 (1 point) Two streams are mixed in an adiabatic mixer, as shown in the figure. It is known that: Stream 1 is saturated
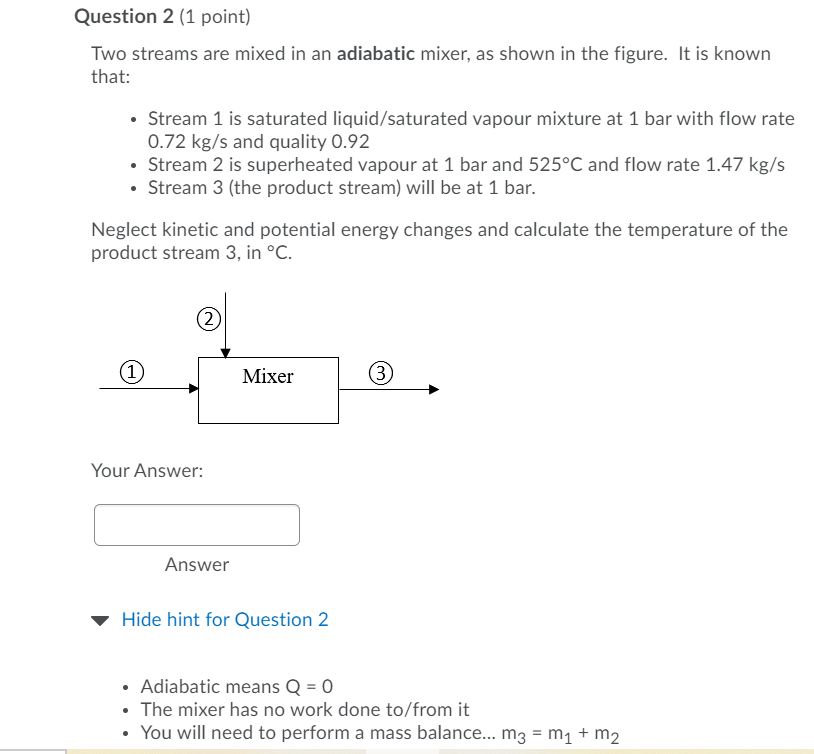
Question 2 (1 point) Two streams are mixed in an adiabatic mixer, as shown in the figure. It is known that: Stream 1 is saturated liquid/saturated vapour mixture at 1 bar with flow rate 0.72 kg/s and quality 0.92 Stream 2 is superheated vapour at 1 bar and 525C and flow rate 1.47 kg/s Stream 3 (the product stream) will be at 1 bar. Neglect kinetic and potential energy changes and calculate the temperature of the product stream 3, in C. 2 (1 Mixer 3 Your Answer: Answer Hide hint for Question 2 Adiabatic means Q = 0 The mixer has no work done to/from it You will need to perform a mass balance... m3 = m + m2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


