Question: I WILL UPVOTE EXAMPLE: 5) A standardized 0.01820.001 M KMnO4 solution were employed to titrate a series of 50.00 ml aliquots of an unknown iron

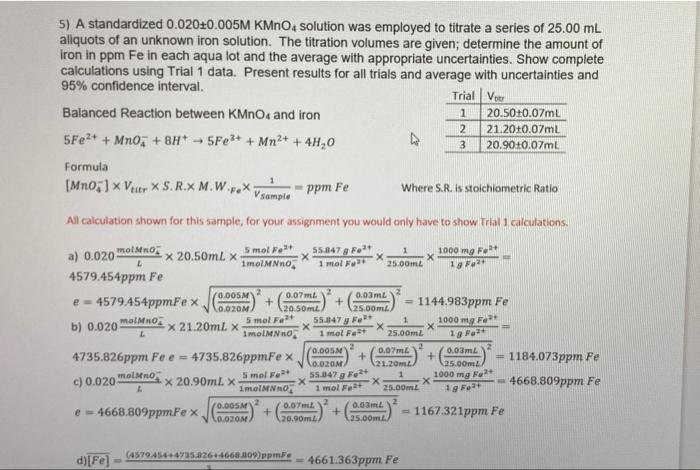
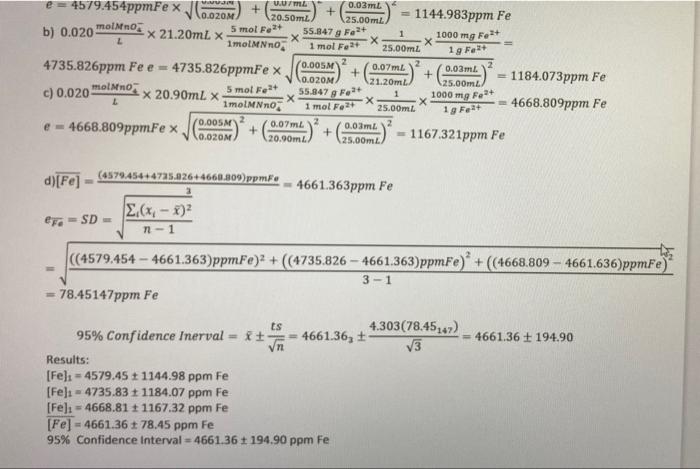
5) A standardized 0.01820.001 M KMnO4 solution were employed to titrate a series of 50.00 ml aliquots of an unknown iron solution. Provide a balanced reaction for the titration from the given half reactions. The titration volumes are given, determine the amount of iron in parts per million (ppm) in each aqua lot and the average with appropriate uncertainties Show complete calculations using Trial 1 data. Present results for all trials and an average with uncertainties Calculate the 95% confidence interval for the final average value Present all results in the table provided. (17 marks) Trial Veer Half Reaction for KMno, and FE? 1 2.95+0.07ml 2 3.1510.07ml Mno: +8H+ +5e Mn2+ + 47,0 Fe2+ Fe3+ + 3 3.45+0.07ml titr 5) A standardized 0.020+0.005M KMnO solution was employed to titrate a series of 25.00 mL aliquots of an unknown iron solution. The titration volumes are given; determine the amount of iron in ppm Fe in each aqua lot and the average with appropriate uncertainties. Show complete calculations using Trial 1 data. Present results for all trials and average with uncertainties and 95% confidence interval. Trial Ve Balanced Reaction between KMnO4 and iron 1 20.50+0.07m. 2 21.20+0.07ml 5Fe2+ + Mnoz +8H+ 5Fe3+ + Mn2+ + 4H20 3 20.90+0.07ml Formula [Mno, ] Vitr X S.R.X M.W.7.* Vsample X ppm Fe Where S.R. is stoichiometric Ratio All calculation shown for this sample, for your assignment you would only have to show Trial calculations, a) 0.020 molano: x 20.50ml. Smol." 55.847 g Fett 1000 mg F L 1molMNO 1 mol Fatt 25.00ml 19 4579.454ppm Fe (0.005 e = 4579.454ppmFex 0.07m. + 0.03 m. 0.020 20.50ml 25.00m 1144.983ppm Fe 5 mol Font X21.20ml x 55.847 Ft 1 1000 mg X ImolMNO 1 mol Fast 25.00ml 0.00M2 0.07m 4735.826ppm Fee = 4735.826ppmFex + + 0.03m. V.OZOM 21.20m 1184.073ppm Fe 25.00m molinos c) 0.020 Smal Fat 1 x 20.90mL X 55.847 Fot X 1000 mg Folt E X Imolano 1 mol For 25.00m2 4668.809ppm Fe 0.COM e-4668.809ppmFex 0.07m 0.03m. + + 0.00M 20.90m 25.00m = 1167.321ppm Fe b) 0.020 molino 1 gr d)[Fe] (487045-14725.2264668.209) ppmF = 4661.363ppm Fe b) 0.020 molano, UUML 0.03 mL e 4579.454ppmFex JG 10.OZOM + 20.50ml 1144.983ppm Fe 125.00 m2 X 21.20mL X 5 mol Fe2+ 55.847 g Folt 1 1molno 1000 mg Fest X 1 mol Fo2+ 25.00ml 19 Fe2+ 4735.826ppm Fee = 2 4735.826ppmFe x 0.005 + 0.07ml + 0.03m 0.020M 21.20m 1184.073ppm Fe 25.00m c) 0.020 molino x 20.90ml x 5 mol Font 55.847 g Feat 1 L 1000 mg Feat ImolMNno X 1 mol Fe2+ 25.00mt 4668.809ppm Fe 0.005 e- 0.07m 2 4668.809ppmFe x + 0.03 0.020M + 20.90m2 1167.321ppm Fe 25.00m 19 Fe+ d)[Fe] (4579.454+4725.9264660.809 ppm. 4661.363ppm Fe EF-SD- 2(x - 2) 1 - 1 ((4579.454 - 4661.363)ppmFe)2 + ((4735.826 - 4661.363)ppmFe) * + (4668.809 - 4661.636)ppmfe) 3-1 78.45147ppm Fe ts 4.303(78.45:47) 95% Confidence Inerval= * = 4661.36, + = 4661.36 + 194.90 vn V3 Results: [Fe) = 4579.45 + 1144.98 ppm Fe [Fe] =4735.83 1184.07 ppm Fe [Fel: = 4668.81 1167.32 ppm Fe [Fe] - 4661.361 78.45 ppm Fe 95% Confidence interval=4661.36 + 194.90 ppm Fe
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


