Question: Question 2 10 points A 27.6 ml sample of PH3(g) (used in the manufacture of flame-retardant chemicals) is obtained at STP. (P:31g/mol; H: 1g/mol) (in
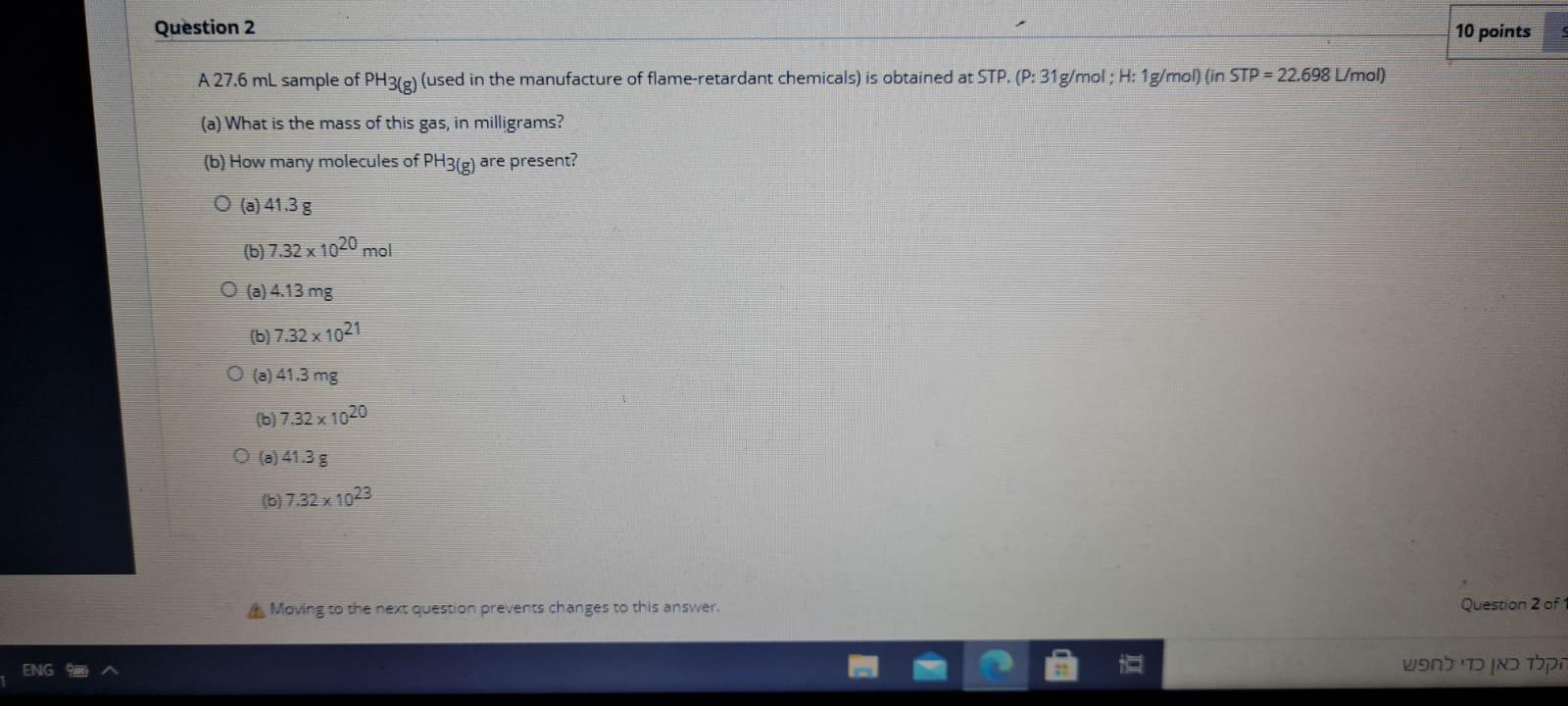
Question 2 10 points A 27.6 ml sample of PH3(g) (used in the manufacture of flame-retardant chemicals) is obtained at STP. (P:31g/mol; H: 1g/mol) (in STP = 22.698 L/mol) (a) What is the mass of this gas, in milligrams? (b) How many molecules of PH3(g) are present? 0 (a) 41.35 (b) 7.32 x 1020 mol O (a) 4.13 mg (b) 7.32 x 1021 (a) 41.3 mg (6) 7.32 x 1020 (a) 41:38 (6) 7.32x 1023 Moving to the next question prevents changes to this answer. Question 2 of 1 ENG
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


