Question: QUESTION 2 (15 marks) Cyclohexene (C6H10) is produced from the elementary reaction of 1,3-butadiene (C4H6) and ethylene (C2H4), C4H + C2H4 C6H10, in a constant
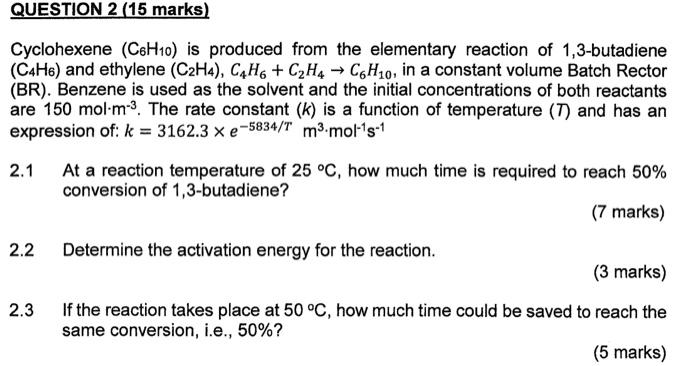
QUESTION 2 (15 marks) Cyclohexene (C6H10) is produced from the elementary reaction of 1,3-butadiene (C4H6) and ethylene (C2H4), C4H + C2H4 C6H10, in a constant volume Batch Rector (BR). Benzene is used as the solvent and the initial concentrations of both reactants are 150 mol-m-3. The rate constant (k) is a function of temperature (T) and has an expression of: k = 3162.3 x e-5834/T m3.mol-15-1 2.1 At a reaction temperature of 25 C, how much time is required to reach 50% conversion of 1,3-butadiene? (7 marks) 2.2 Determine the activation energy for the reaction. (3 marks) 2.3 If the reaction takes place at 50 C, how much time could be saved to reach the same conversion, i.e., 50%
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


