Question: QUESTION 2 : ( 3 0 pts ) : Molecular weight averages and distribution, characterizations The most important feature of polymers compared to small molecules
QUESTION : pts: Molecular weight averages and distribution, characterizations
The most important feature of polymers compared to small molecules is their sizes. The sizes
of the macromolecules primarily determine their physical properties. When we say size, we
may refer to either the degree of polymerization molecular weights or the spatial arrangement
of the polymers which gives its actual shape in D The synthetic polymers come with a
distribution of molecular weights, and it is important to know the average and the dispersity.
a pts Show that where is the weight fraction of chains with molar
mass and is the number average molecular weight.
b pts Four samples of a polymer is mixed without reaction. Each individual sample
has narrow molecular weight distribution. Calculate the number and weight average
molecular weights of the mixture and the resulting dispersity.
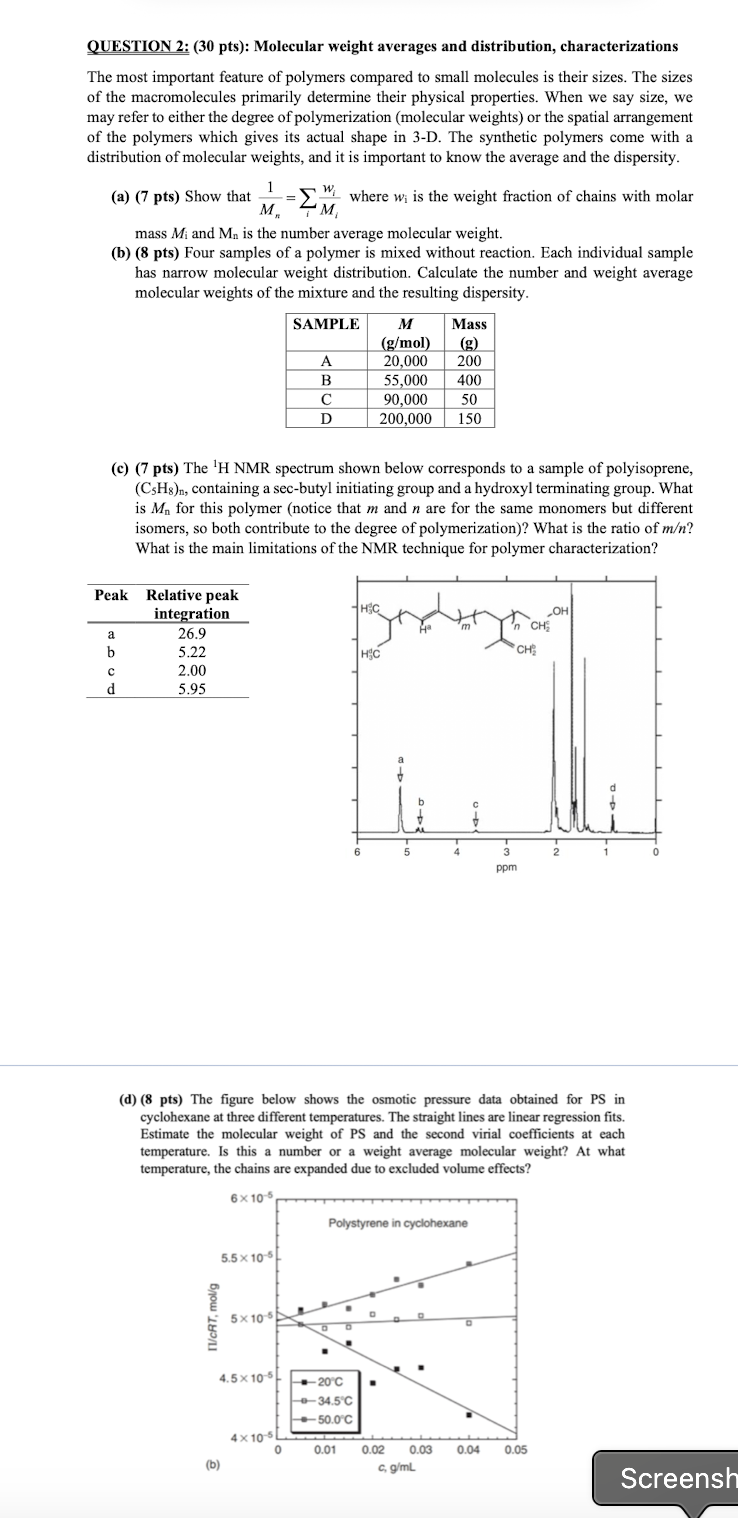
c pts The NMR spectrum shown below corresponds to a sample of polyisoprene,
containing a secbutyl initiating group and a hydroxyl terminating group. What
is for this polymer notice that and are for the same monomers but different
isomers, so both contribute to the degree of polymerization What is the ratio of
What is the main limitations of the NMR technique for polymer characterization?
d pts The figure below shows the osmotic pressure data obtained for PS in
cyclohexane at three different temperatures. The straight lines are linear regression fits.
Estimate the molecular weight of PS and the second virial coefficients at each
temperature. Is this a number or a weight average molecular weight? At what
temperature, the chains are expanded due to excluded volume effects?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


