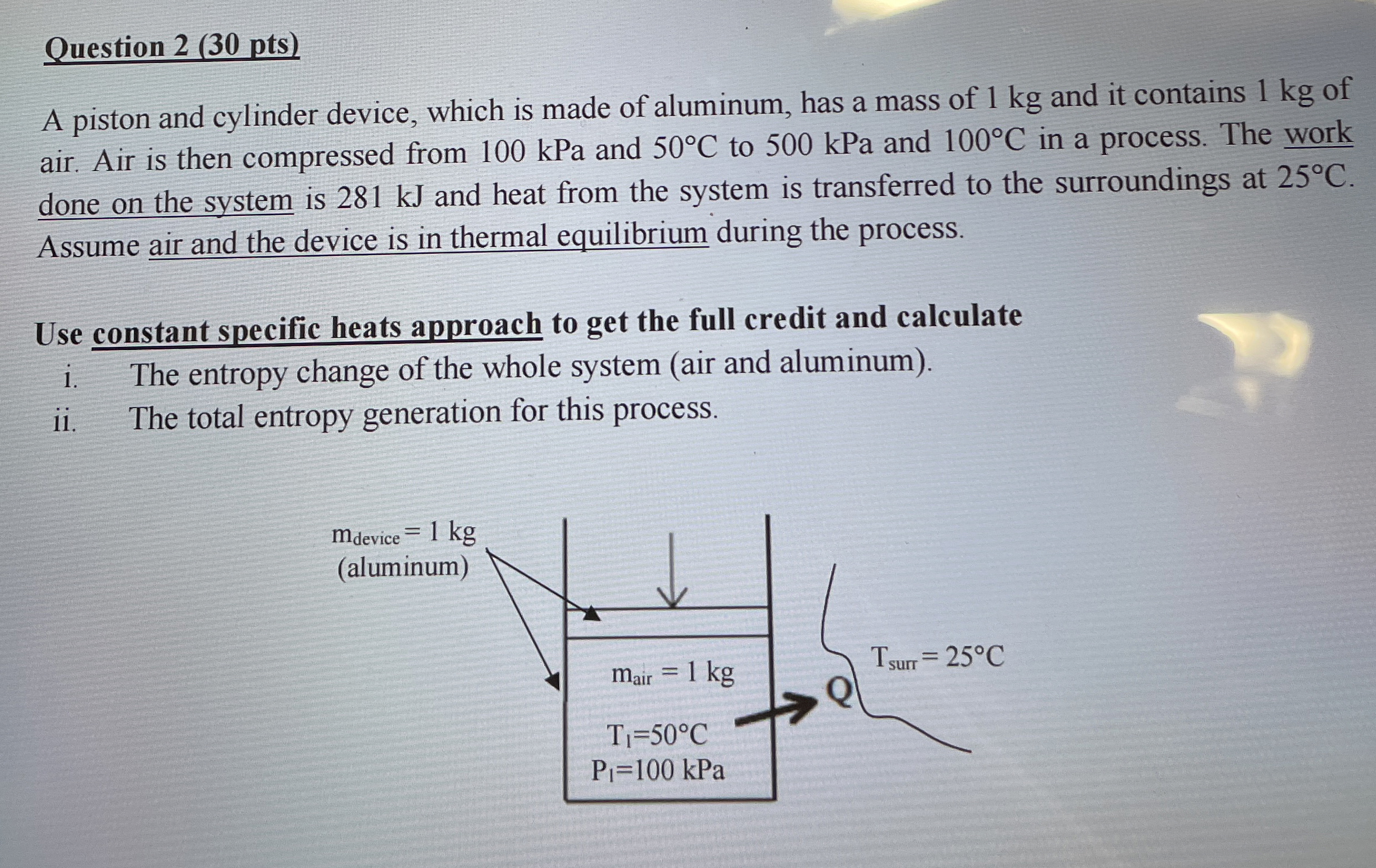
Question: Question 2 ( 3 0 pts ) ( please solve step by step ) A piston and cylinder device, which is made of aluminum, has
Question ptsplease solve step by step
A piston and cylinder device, which is made of aluminum, has a mass of kg and it contains kg of air. Air is then compressed from kPa and to kPa and in a process. The work done on the system is kJ and heat from the system is transferred to the surroundings at Assume air and the device is in thermal equilibrium during the process.
Use constant specific heats approach to get the full credit and calculate
i The entropy change of the whole system air and aluminum
ii The total entropy generation for this process.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


