Question: Question #2: (35 Marks) Nitrogen dioxide can dimerize to powerful nitrogen tetroxide: 2NO, N20, which is used with a liquid fuel as propellant in rockets.
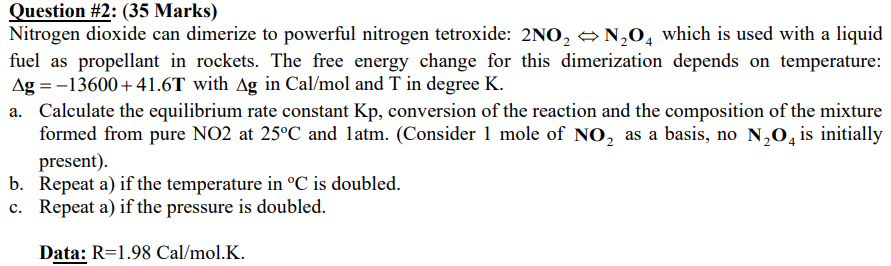
Question #2: (35 Marks) Nitrogen dioxide can dimerize to powerful nitrogen tetroxide: 2NO, N20, which is used with a liquid fuel as propellant in rockets. The free energy change for this dimerization depends on temperature: Ag =-13600+41.6T with Ag in Cal/mol and T in degree K. a. Calculate the equilibrium rate constant Kp, conversion of the reaction and the composition of the mixture formed from pure NO2 at 25C and latm. (Consider 1 mole of NO, as a basis, no N20, is initially present). b. Repeat a) if the temperature in C is doubled. c. Repeat a) if the pressure is doubled. Data: R=1.98 Cal/mol.K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


