Question: Question 2 (5 marks) SO2 is absorbed from air by water in a packed column at 20 C. At a certain location, the total pressure
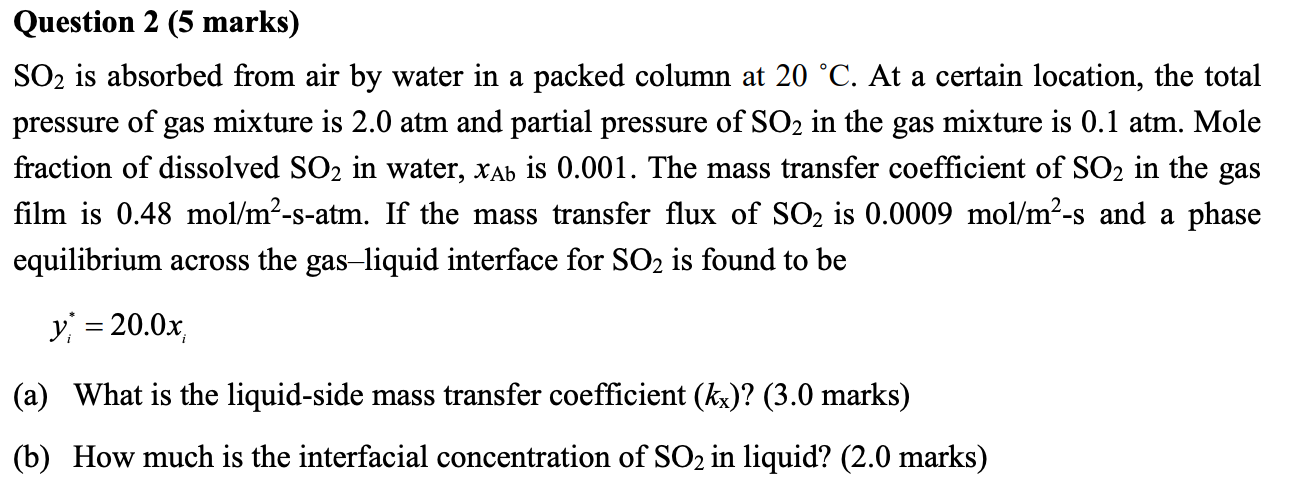
Question 2 (5 marks) SO2 is absorbed from air by water in a packed column at 20 C. At a certain location, the total pressure of gas mixture is 2.0 atm and partial pressure of SO2 in the gas mixture is 0.1 atm. Mole fraction of dissolved SO2 in water, Xab is 0.001. The mass transfer coefficient of SO2 in the gas film is 0.48 mol/m2-s-atm. If the mass transfer flux of SO2 is 0.0009 mol/m-s and a phase equilibrium across the gas-liquid interface for SO2 is found to be y; = 20.0x (a) What is the liquid-side mass transfer coefficient (kx)? (3.0 marks) (b) How much is the interfacial concentration of SO2 in liquid? (2.0 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


