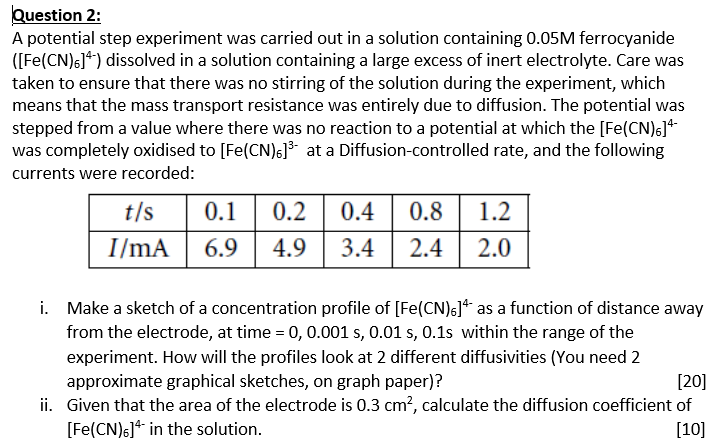
Question: Question 2 : A potential step experiment was carried out in a solution containing 0 . 0 5 M ferrocyanide ( [ F e (
Question :
A potential step experiment was carried out in a solution containing ferrocyanide
dissolved in a solution containing a large excess of inert electrolyte. Care was
taken to ensure that there was no stirring of the solution during the experiment, which
means that the mass transport resistance was entirely due to diffusion. The potential was
stepped from a value where there was no reaction to a potential at which the
was completely oxidised to at a Diffusioncontrolled rate, and the following
currents were recorded:
i Make a sketch of a concentration profile of as a function of distance away
from the electrode, at time within the range of the
experiment. How will the profiles look at different diffusivities of cms and cms You need
approximate graphical sketches, on graph paper
ii Given that the area of the electrode is calculate the diffusion coefficient of
in the solution.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


