Question: question 2 and 5 please You carry out Run A based on Table 1 in the lab manual, using 0.0145MNa2S2O3. What is AH2O2l(in molar units)?


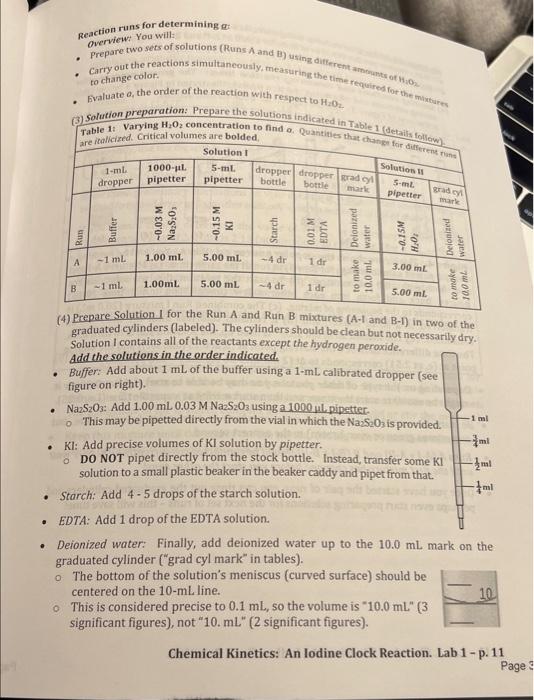
You carry out Run A based on Table 1 in the lab manual, using 0.0145MNa2S2O3. What is AH2O2l(in molar units)? Enter the numerical value, without units, to 3 Mignificant figures. To be counted as correct, your answer must be within 1 w of the properly calculated value, so maintain 3 significant figures in your calculations. Aso, the sign of the answer is important. Enter your answer in standard decimal format (e.g. 0.0123), NOT in selentific notation. A reaction is carried out with [H2O2]=0.000817M, with a reaction time of 4 minutes 59 seconds. What is the reaction rate, with units of M/s? Enter the numerical value in scientific notation to 3 significant figures. Use "f.notation" to enter values in scientific notation , e.g. 1.23104 would be entered as 1.23 E. 4 (no spaces, capital E). To be counted as correct, you answer should be within 19 of the correctly calculated value. Reaction runs for determining at (3) Solution preparation: Prepare the solutions indicated in Table 1 (detait.. (4) Preparesolution. I for the Run A and Run B mixtures (A-l and B1] in two of the graduated cylinders (labeled). The cylinders should be clean but not necessarily dry. Solution 1 contains all of the reactants except the hydrogen peroxide. Add the solutions in the order indicated. - Buffer: Add about 1 mL of the buffer using a 1-mL calibrated dropper (see figure on right). - Na2S2O3: Add 1.00mL0.03MNaS2O3 using a 1000 ul pipetter. Na2S2Os: Add 1.00mL0.03MNaS2O3 using a 1000 ul pipetter. o This may be pipetted directly from the vial in which the NazS2O is provided. KI: Add precise volumes of KI solution by pipetter. DO NOT pipet directly from the stock bottle. Instead, transfer some KI solution to a small plastic beaker in the beaker caddy and pipet from that. Starch: Add 4 - 5 drops of the starch solution. EDTA: Add 1 drop of the EDTA solution. - Deionized water: Finally, add deionized water up to the 10.0mL mark on the graduated cylinder ("grad cyl mark" in tables). - The bottom of the solution's meniscus (curved surface) should be centered on the 10mL line. This is considered precise to 0.1mL, so the volume is 10.0mL " (3 significant figures), not "10. mL" (2 significant figures). Chemical Kinetics: An lodine Clock Reaction. Lab 1 - p. 11 You carry out Run A based on Table 1 in the lab manual, using 0.0145MNa2S2O3. What is AH2O2l(in molar units)? Enter the numerical value, without units, to 3 Mignificant figures. To be counted as correct, your answer must be within 1 w of the properly calculated value, so maintain 3 significant figures in your calculations. Aso, the sign of the answer is important. Enter your answer in standard decimal format (e.g. 0.0123), NOT in selentific notation. A reaction is carried out with [H2O2]=0.000817M, with a reaction time of 4 minutes 59 seconds. What is the reaction rate, with units of M/s? Enter the numerical value in scientific notation to 3 significant figures. Use "f.notation" to enter values in scientific notation , e.g. 1.23104 would be entered as 1.23 E. 4 (no spaces, capital E). To be counted as correct, you answer should be within 19 of the correctly calculated value. Reaction runs for determining at (3) Solution preparation: Prepare the solutions indicated in Table 1 (detait.. (4) Preparesolution. I for the Run A and Run B mixtures (A-l and B1] in two of the graduated cylinders (labeled). The cylinders should be clean but not necessarily dry. Solution 1 contains all of the reactants except the hydrogen peroxide. Add the solutions in the order indicated. - Buffer: Add about 1 mL of the buffer using a 1-mL calibrated dropper (see figure on right). - Na2S2O3: Add 1.00mL0.03MNaS2O3 using a 1000 ul pipetter. Na2S2Os: Add 1.00mL0.03MNaS2O3 using a 1000 ul pipetter. o This may be pipetted directly from the vial in which the NazS2O is provided. KI: Add precise volumes of KI solution by pipetter. DO NOT pipet directly from the stock bottle. Instead, transfer some KI solution to a small plastic beaker in the beaker caddy and pipet from that. Starch: Add 4 - 5 drops of the starch solution. EDTA: Add 1 drop of the EDTA solution. - Deionized water: Finally, add deionized water up to the 10.0mL mark on the graduated cylinder ("grad cyl mark" in tables). - The bottom of the solution's meniscus (curved surface) should be centered on the 10mL line. This is considered precise to 0.1mL, so the volume is 10.0mL " (3 significant figures), not "10. mL" (2 significant figures). Chemical Kinetics: An lodine Clock Reaction. Lab 1 - p. 11
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


