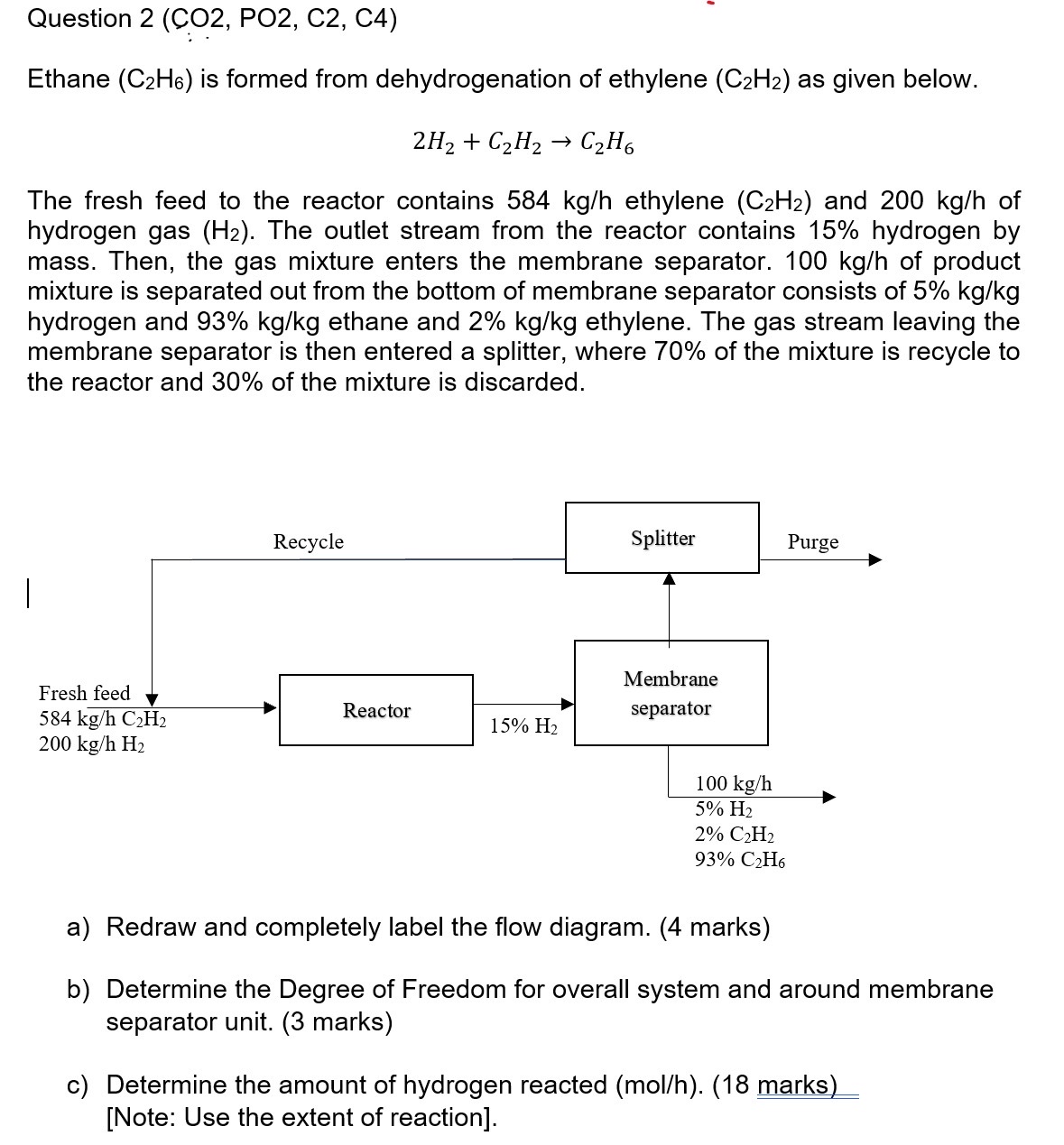
Question: Question 2 ( CO 2 , PO 2 , C 2 , C 4 ) Ethane ( C 2 H 6 ) is formed from
Question CO PO C C
Ethane is formed from dehydrogenation of ethylene as given below.
The fresh feed to the reactor contains ethylene and of hydrogen gas The outlet stream from the reactor contains hydrogen by mass. Then, the gas mixture enters the membrane separator. of product mixture is separated out from the bottom of membrane separator consists of hydrogen and ethane and ethylene. The gas stream leaving the membrane separator is then entered a splitter, where of the mixture is recycle to the reactor and of the mixture is discarded.
a Redraw and completely label the flow diagram. marks
b Determine the Degree of Freedom for overall system and around membrane separator unit. marks
c Determine the amount of hydrogen reacted mol marksNote: Use the extent of reaction
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


