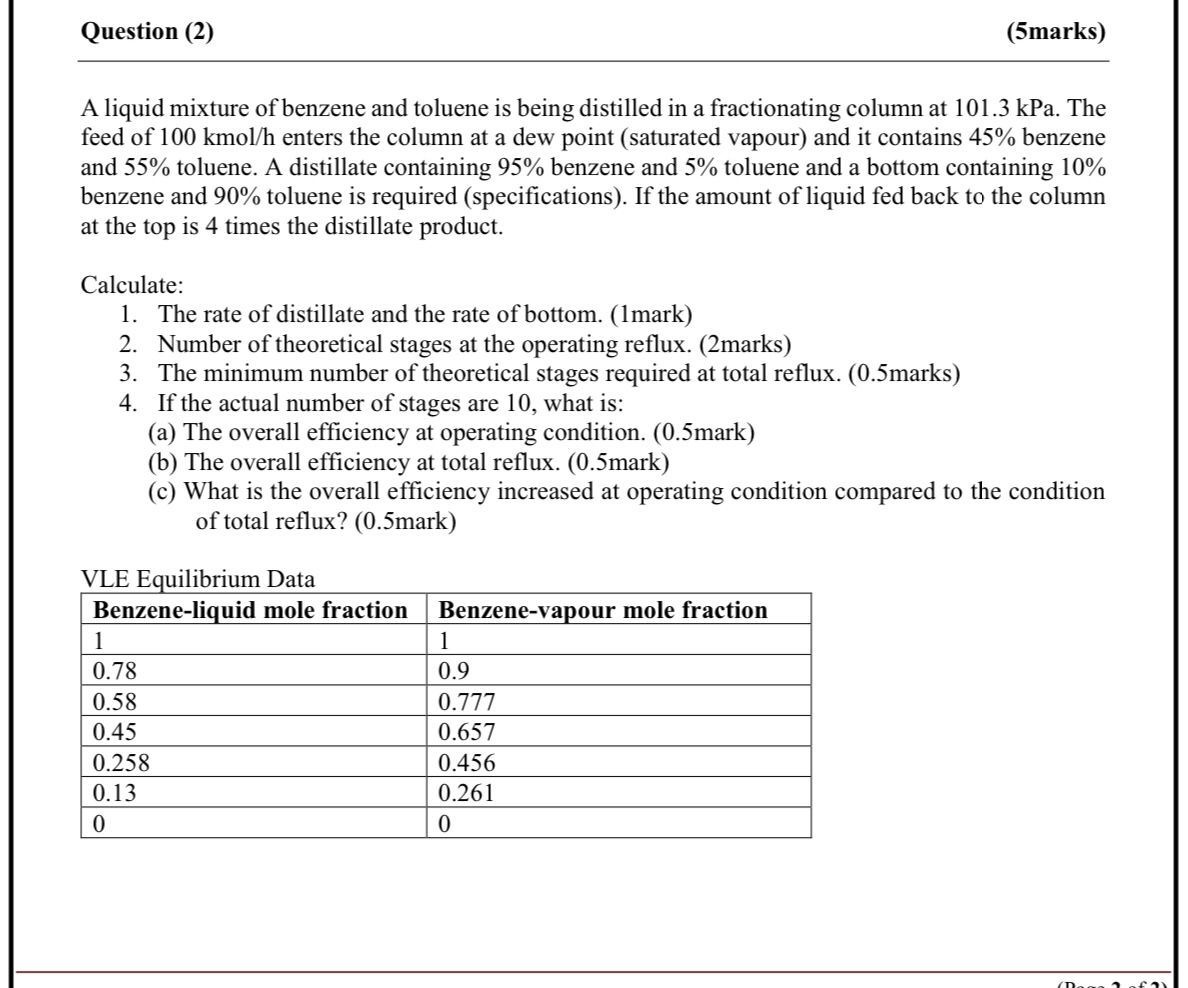
Question: Question ( 2 ) : please explain all the steps and draw on paper. ( 5 marks ) A liquid mixture of benzene and toluene
Question : please explain all the steps and draw on paper.
marks
A liquid mixture of benzene and toluene is being distilled in a fractionating column at kPa. The feed of kmo enters the column at a dew point saturated vapour and it contains benzene and toluene. A distillate containing benzene and toluene and a bottom containing benzene and toluene is required specifications If the amount of liquid fed back to the column at the top is times the distillate product.
Calculate:
The rate of distillate and the rate of bottom. mark
Number of theoretical stages at the operating reflux. marks
The minimum number of theoretical stages required at total reflux. marks
If the actual number of stages are what is:
a The overall efficiency at operating condition. mark
b The overall efficiency at total reflux. mark
c What is the overall efficiency increased at operating condition compared to the condition of total reflux? mark
VLE Equilibrium Data
tableBenzeneliquid mole fraction,Benzenevapour mole fraction
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


