Question: QUESTION 2 (PO2, CO3, C6) A process for ethanol synthesis has been developed and the ethylene hydration operating conditions have been optimized in order to
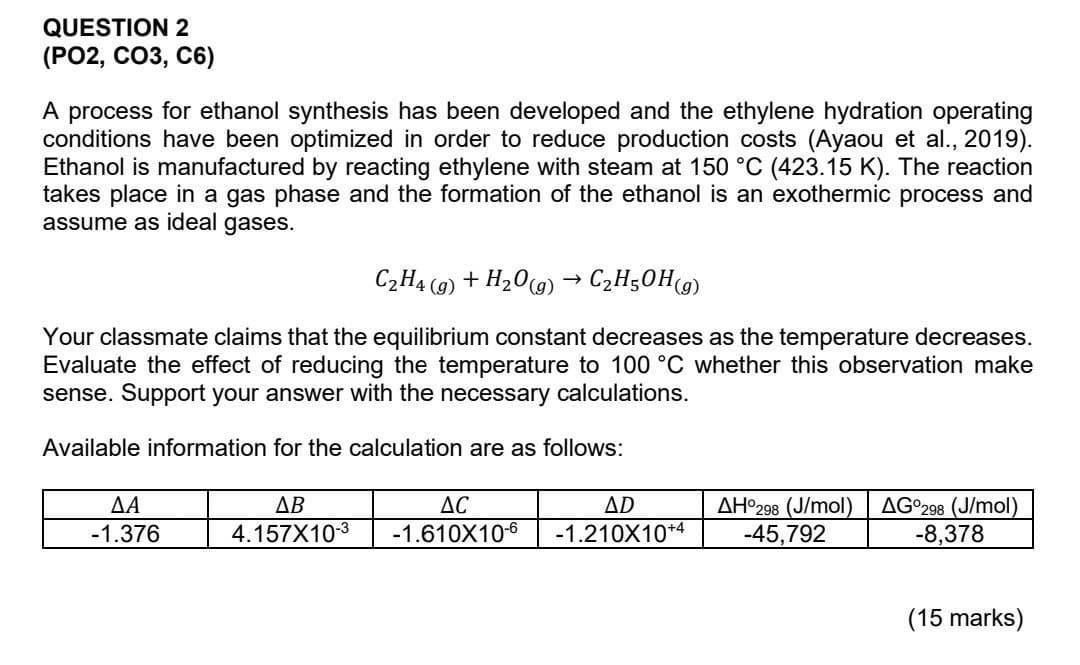
QUESTION 2 (PO2, CO3, C6) A process for ethanol synthesis has been developed and the ethylene hydration operating conditions have been optimized in order to reduce production costs (Ayaou et al., 2019). Ethanol is manufactured by reacting ethylene with steam at 150 C (423.15 K). The reaction takes place in a gas phase and the formation of the ethanol is an exothermic process and assume as ideal gases. C2H4 (9) + H20(g) C2H50Hg) + Your classmate claims that the equilibrium constant decreases as the temperature decreases. Evaluate the effect of reducing the temperature to 100 C whether this observation make sense. Support your answer with the necessary calculations. Available information for the calculation are as follows: -1.376 AB 4.157X10-3 AC -1.610X10-6 AD -1.210X10+4 AH298 (J/mol) -45,792 AG298 (J/mol) -8,378 (15 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


