Question: QUESTION 2 Using a ceramic electrolyte, a fuel cell can operate at 827 C. Pure oxygen is used as the oxidizer. Both CO and oxygen
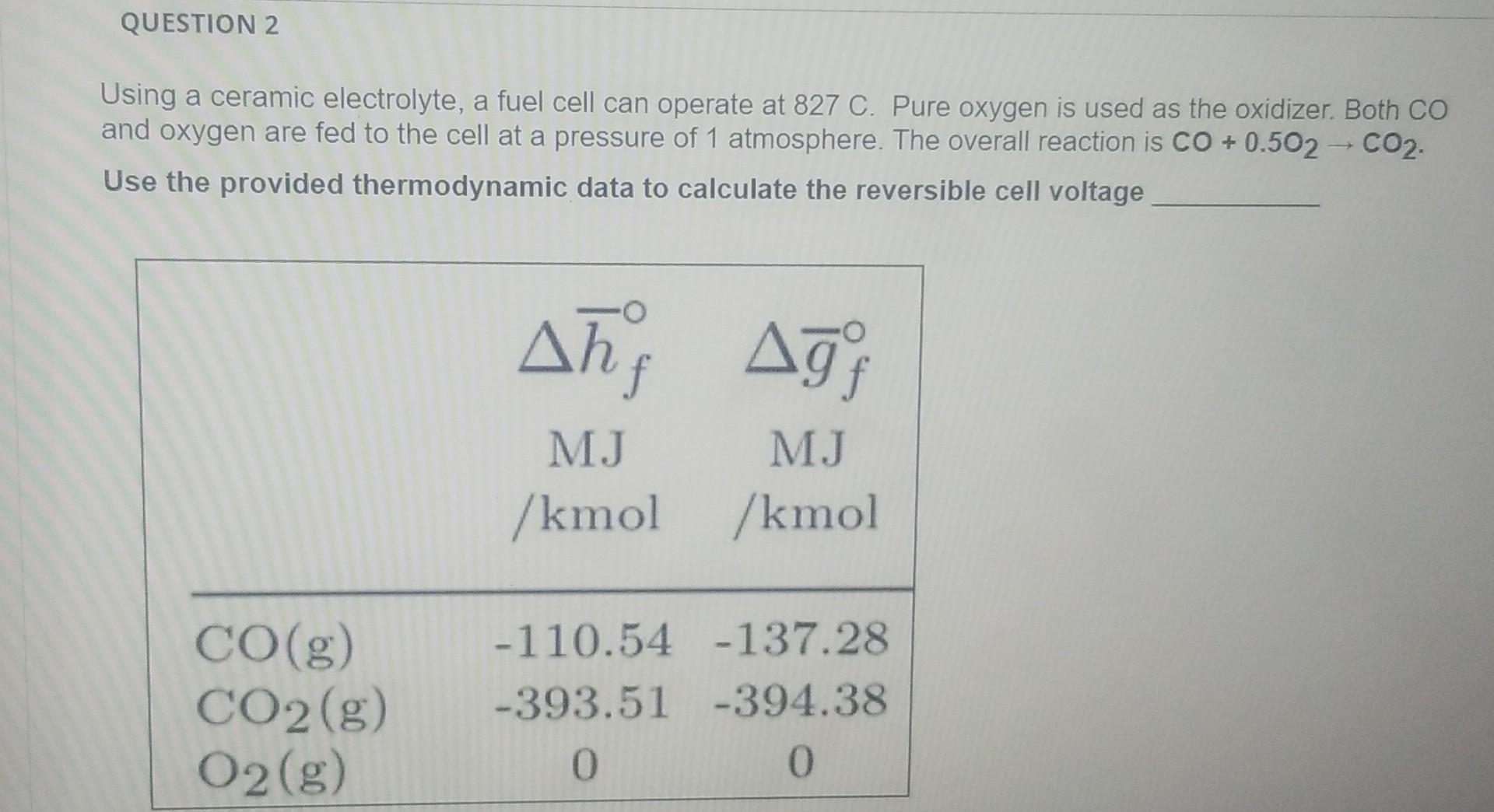
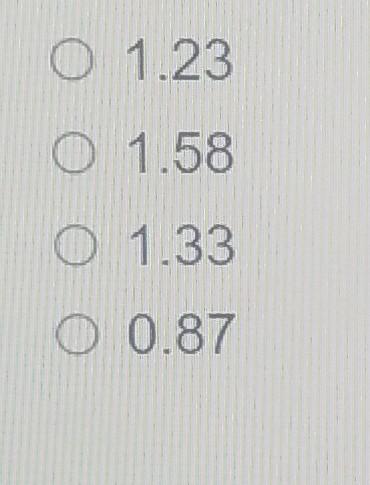

QUESTION 2 Using a ceramic electrolyte, a fuel cell can operate at 827 C. Pure oxygen is used as the oxidizer. Both CO and oxygen are fed to the cell at a pressure of 1 atmosphere. The overall reaction is CO + 0.502 - CO2. Use the provided thermodynamic data to calculate the reversible cell voltage Ah; Ag) MJ MJ /kmol /kmol CO(g) CO2(g) O2(g) -110.54 -137.28 -393.51 -394.38 0 O O 1.23 0 1.58 O 1.33 O 0.87 Match the different regions labeled in the following fuel cell polarization curve
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


