Question: Question 21 [4 points) Years ago a high school physics teacher put some gas tubes into storage but forgot to label them. Now the school
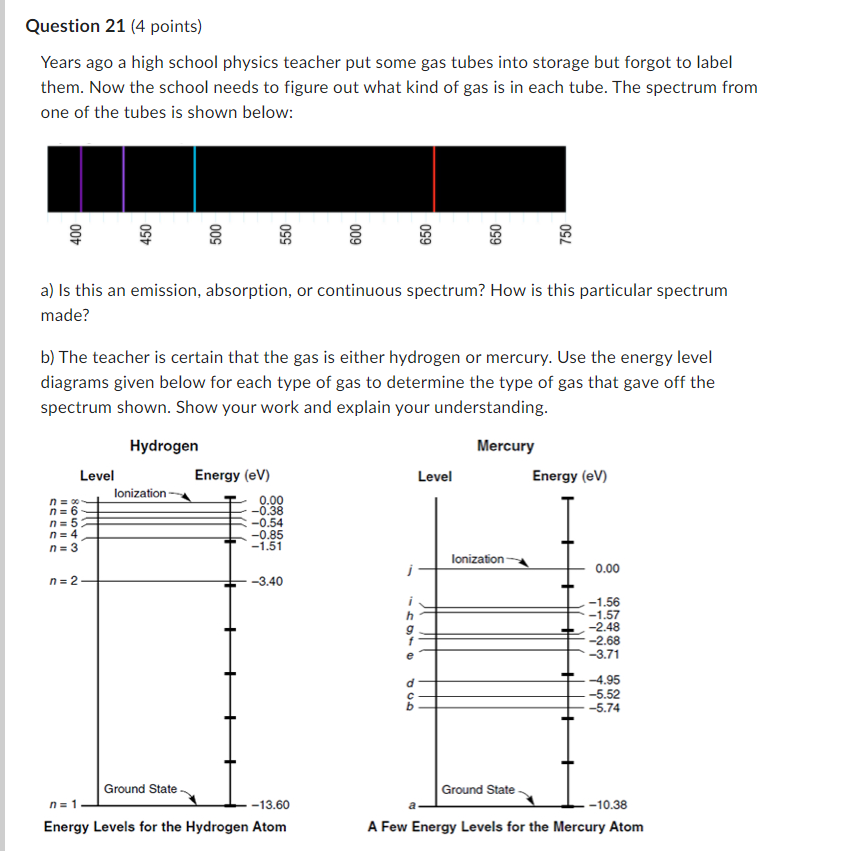
Question 21 [4 points) Years ago a high school physics teacher put some gas tubes into storage but forgot to label them. Now the school needs to figure out what kind of gas is in each tube. The spectrum from one of the tubes is shown below: 3 E E E S E E E u- 'EI' u'J u'J cc: co to h. a} Is this an emission. absorption, or continuous spectrum? How is this particular spectrum made? b} The teacher is certain that the gas is either hydrogen or mercury. Use the energy level diagrams given below for each type of gas to determine the type of gas that gave off the spectrum shown. Show your work and explain your understanding. Hydrogen Mercury Lave] Energy {elf} Level Energy [er n : x Ionization _ 000 n = 6 = 4.38 H = 5 '- O.54 n = 4 -035 n = 3 l .51 j 0.00 n = 2 4.40 r' 4.56 h ' l.5? g _ 2.43 f 4.68; e ' -3.?1 d 41.95 c: -5.52 b 5.?4 Ground Stale. n = I 43.60 a 40.38 Energy Levels {our the Hydrogen Atom A Few Energy Levels for the Mercury Atom
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


