Question: Question 3 1 pts Look at sample problem 17.10 in the 8th edition Silberberg book. The research and development unit of a chemical company is
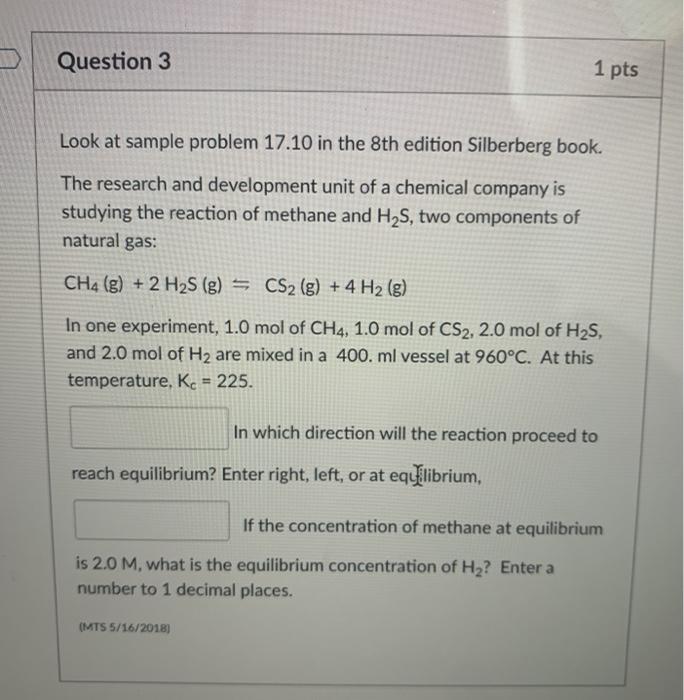
Question 3 1 pts Look at sample problem 17.10 in the 8th edition Silberberg book. The research and development unit of a chemical company is studying the reaction of methane and H2S, two components of natural gas: CH4 (g) + 2 H2S (g) = CS2 (g) + 4 H2 (g) In one experiment, 1.0 mol of CH4, 1.0 mol of CS2, 2.0 mol of H2S, and 2.0 mol of H2 are mixed in a 400. ml vessel at 960C. At this temperature. Kc = 225. In which direction will the reaction proceed to reach equilibrium? Enter right, left, or at equilibrium, If the concentration of methane at equilibrium is 2.0 M, what is the equilibrium concentration of H2? Enter a number to 1 decimal places. (MTS 5/16/2018)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


