Question: Question 3 (10 points) If one can find the ratio of the number of moles of the elements in a compound to one another, one
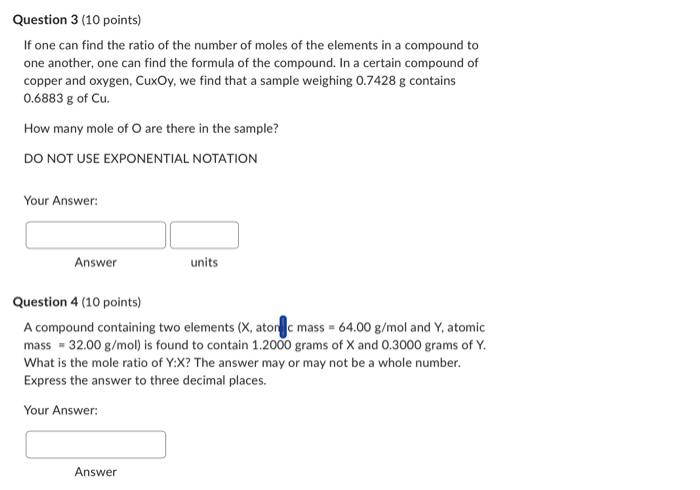

Question 3 (10 points) If one can find the ratio of the number of moles of the elements in a compound to one another, one can find the formula of the compound. In a certain compound of copper and oxygen, CuxOy, we find that a sample weighing 0.7428g contains 0.6883g of Cu. How many mole of O are there in the sample? DO NOT USE EXPONENTIAL NOTATION Your Answer: Answer units Question 4 (10 points) A compound containing two elements (X, atonf c mass =64.00g/mol and Y, atomic mass =32.00g/mol ) is found to contain 1.2000 grams of X and 0.3000 grams of Y. What is the mole ratio of Y:X ? The answer may or may not be a whole number. Express the answer to three decimal places. Your Answer: Answer A compound containing two elements (x, atomic mass =04.g/mol and Y, atomic mass =32.00g/mol ) is found to contain 1.2000 grams of X and 0.3000grams of Y. What is the mole ratio of Y:X ? The answer may or may not be a whole number. Express the answer to three decimal places. Your Answer: Answer Question 5 (10 points) A carbohydrate is found to contain 0.2000 grams of C and 0.1500 grams of water. What is the mole ratio of C:water? The answer may or may not be a whole number. Express the answer to three decimal places. Your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


