Question: Question 3 2 Points A combustion reaction has reactants 1 mole of CxHy and 2 moles of O2 and products 1 mole of CO2 and
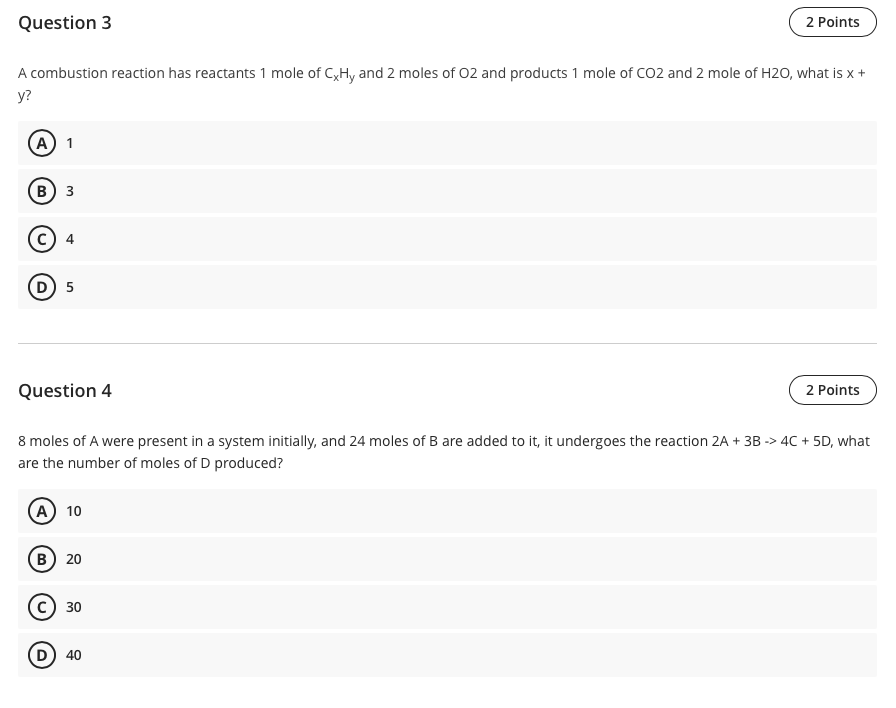
Question 3 2 Points A combustion reaction has reactants 1 mole of CxHy and 2 moles of O2 and products 1 mole of CO2 and 2 mole of H20, what is x + y? A 1 B 3 4 D) 5 Question 4 2 Points 8 moles of A were present in a system initially, and 24 moles of B are added to it, it undergoes the reaction 2A + 3B -> 4C + 5D, what are the number of moles of D produced? A 10 B 20 C 30 D) 40
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


