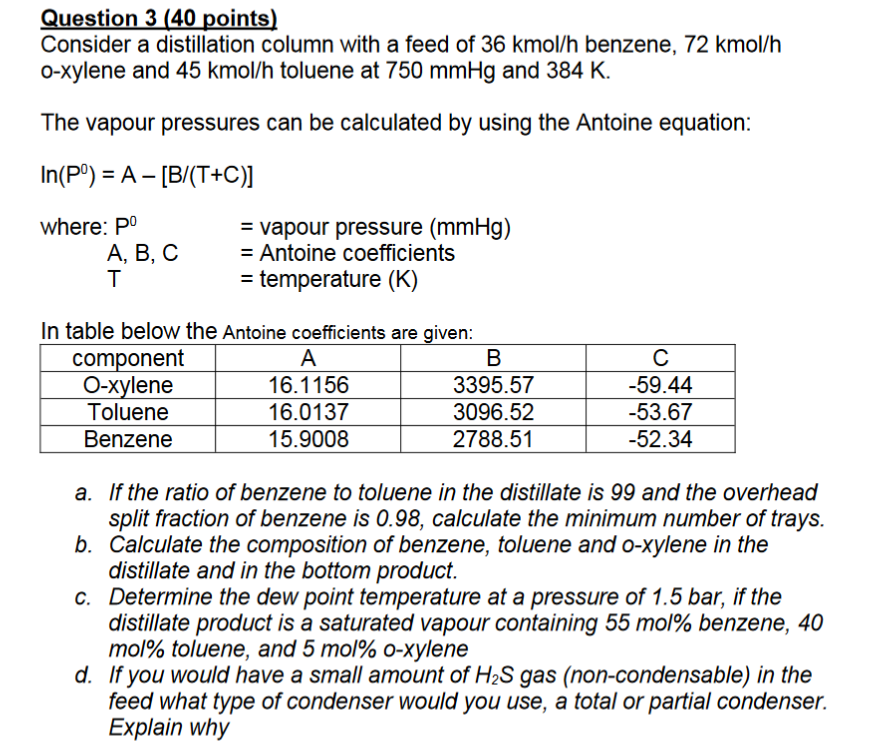
Question: Question 3 ( 4 0 points ) Consider a distillation column with a feed of 3 6 kmo l h benzene, 7 2 kmo l
Question points
Consider a distillation column with a feed of kmo benzene, kmo
oxylene and kmo toluene at and
The vapour pressures can be calculated by using the Antoine equation:
where: vapour pressure
Antoine coefficients
temperature
In table below the Antoine coefficients are given:
a If the ratio of benzene to toluene in the distillate is and the overhead
split fraction of benzene is calculate the minimum number of trays.
b Calculate the composition of benzene, toluene and oxylene in the
distillate and in the bottom product.
c Determine the dew point temperature at a pressure of bar, if the
distillate product is a saturated vapour containing mol benzene,
mol toluene, and mol oxylene
d If you would have a small amount of gas noncondensable in the
feed what type of condenser would you use, a total or partial condenser.
Explain why
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


