Question: Question 3. Determine the correct structure of an ether from the spectra below and explain how you made your conclusion. No solvent peaks can be
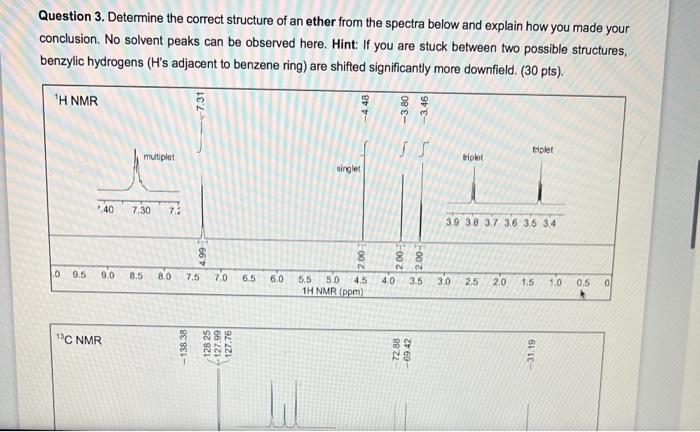
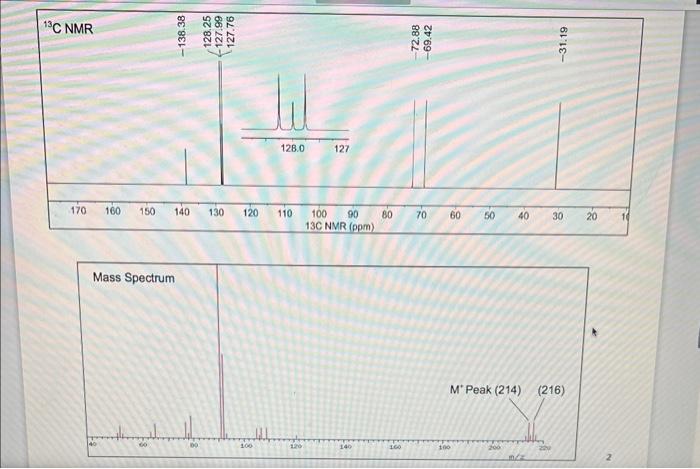

Question 3. Determine the correct structure of an ether from the spectra below and explain how you made your conclusion. No solvent peaks can be observed here. Hint: If you are stuck between two possible structures, benzylic hydrogens ( H 's adjacent to benzene ring) are shifted significantly more downfield. ( 30pts). Question 4. You are trying to determine the identity of a compound that was purified. Use the following 1H NMR, 13CNMR,IR, and MS data to determine the correct compound. Hint: the compound is still "wet" with ethyl acetate and hexanes as solvents, so make sure to identify the solvent peaks first before determining the structure. (50 pts total for the question) Be sure to provide the structure of the compound (15 pts), a properly integrated 1H NMR with all solvent peaks labelled correctly (15 pts), and explain how you determined the structure from the 1H NMR (5pts),13C NMR ( 5 pts), MS ( 5 pts) and IR ( 5 pts). 1H NMR and 10C NMR MNova file: Lab4_NMR_Unknown IR and MS PDF file: Lab4_MS_IR_Unknown
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


