Question: Question 3 i. Write the balanced equation for stoichiometric combustion of Dodecane, C12H26. Assume 21% Oxygen and 79 % Nitrogen in air. Atomic Mass: Carbon
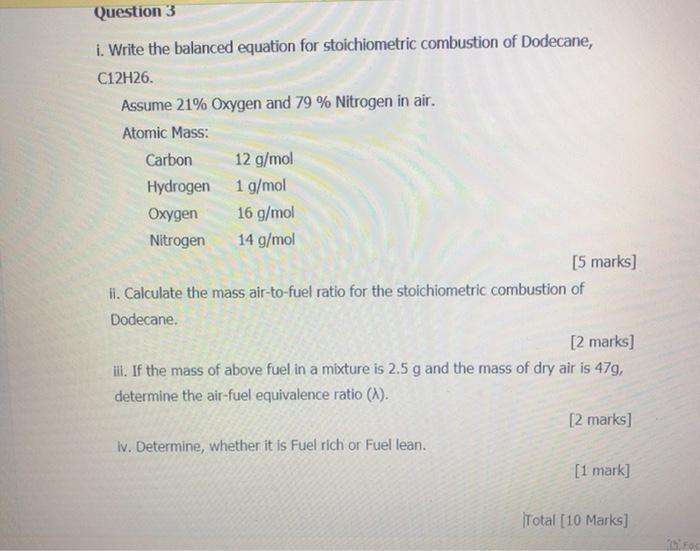
Question 3 i. Write the balanced equation for stoichiometric combustion of Dodecane, C12H26. Assume 21% Oxygen and 79 % Nitrogen in air. Atomic Mass: Carbon 12 g/mol Hydrogen 1 g/mol Oxygen 16 g/mol Nitrogen 14 g/mol [5 marks] ii. Calculate the mass air-to-fuel ratio for the stoichiometric combustion of Dodecane. [2 marks] ill. If the mass of above fuel in a mixture is 2.5 g and the mass of dry air is 479, determine the air-fuel equivalence ratio (~). [2 marks] iv. Determine, whether it is Fuel rich or Fuel lean. [1 mark] Total [10 Marks]
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


