Question: *Question 3 options: acidic solution or basic solution. Zoom in with Ctrl + in order to see the question, I can't make it any bigger.

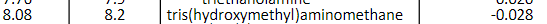
*Question 3 options: acidic solution or basic solution.
Zoom in with Ctrl + in order to see the question, I can't make it any bigger.
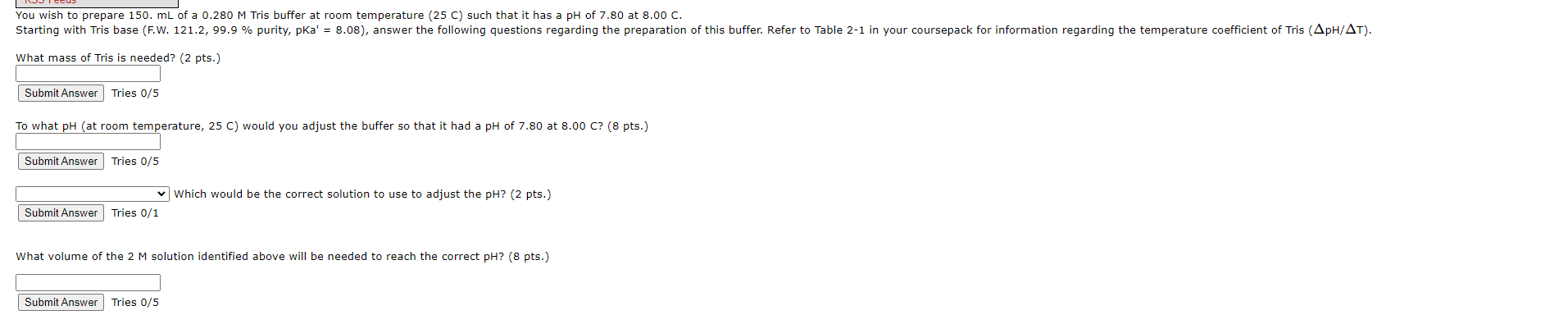
You wish to prepare 150.mL of a 0.280M Tris buffer at room temperature (25 C) such that it has a pH of 7.80 at 8.00C. What mace nf Tric is nootad? (2 pts.) Tries 0/5 To what pH (at room temperature, 25C ) would you adjust the buffer so that it had a pH of 7.80 at 8.00C ? ( 8pts ) Tries 0/5 Which would be the correct solution to use to adjust the pH? (2 pts.) Tries 0/1 What volume of the 2M solution identified above will be needed to reach the correct pH? (8 pts.) Tries 0/5 \begin{tabular}{|c|c|c|c|} \hline pKa at 25 & pKa & Compound & pH/t(pH units per C) \\ \hline \end{tabular} \begin{tabular}{l|l|l|l} 8.08 & 8.2 & tris(hydroxymethyl)aminomethane & 0.028 \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


