Question: Question 3: Redox Titration (11 points) Use the image below to answer the following questions about redox titrations. a. Write 23 sentences describing and identifying
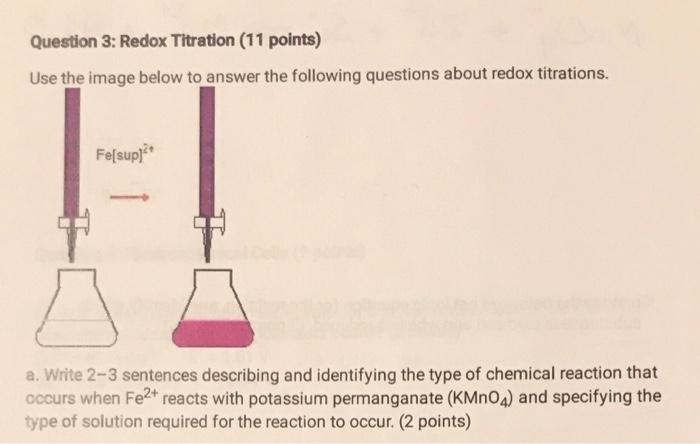



Question 3: Redox Titration (11 points) Use the image below to answer the following questions about redox titrations. a. Write 23 sentences describing and identifying the type of chemical reaction that occurs when Fe2+ reacts with potassium permanganate (KMnO4) and specifying the type of solution required for the reaction to occur. (2 points) b. Using a standard reduction potential table, identify the oxidation and reduction half-reactions for the reaction. ( 4 points) c. Write the balanced net ionic equation for the reaction, and identify which substance is oxidized and which is reduced. (3 points) d. Suppose at the endpoint of the reaction 0.030 moles of KMnO4 were added to the analyte. How many moles of Fe2+ were contained in the beaker? (2 points)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


