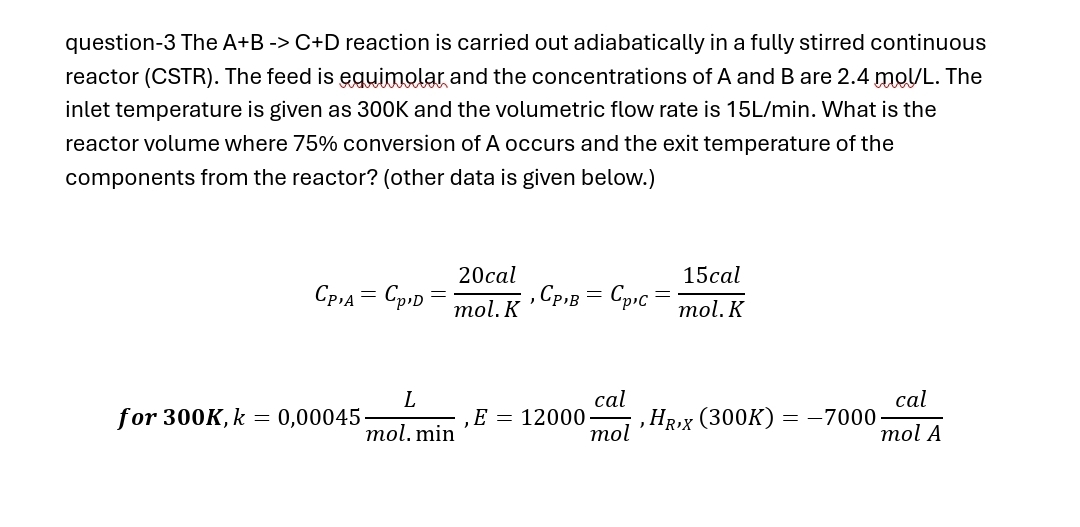
Question: question - 3 The A + B - > C + D reaction is carried out adiabatically in a fully stirred continuous reactor ( CSTR
question The reaction is carried out adiabatically in a fully stirred continuous reactor CSTR The feed is equimolar and the concentrations of A and are The inlet temperature is given as and the volumetric flow rate is What is the reactor volume where conversion of A occurs and the exit temperature of the components from the reactor? other data is given below.
for
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


