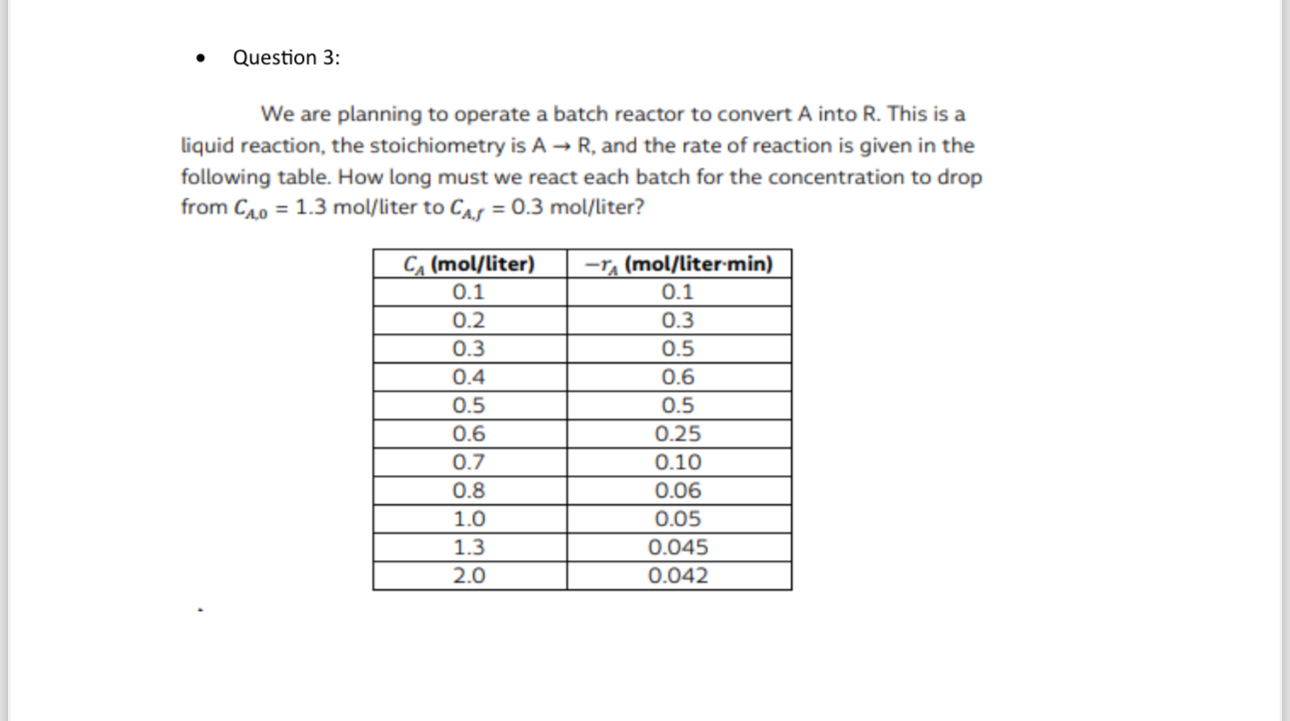
Question: Question 3 : We are planning to operate a batch reactor to convert A into R . This is a liquid reaction, the stoichiometry is
Question :
We are planning to operate a batch reactor to convert A into This is a liquid reaction, the stoichiometry is and the rate of reaction is given in the following table. How long must we react each batch for the concentration to drop from liter to liter?
tablemollitermolliter :
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


