Question: Question 4 (0.5 points) The gas equation for one mole of oxygen related its pressure P [in atmospheres), its temperature, T (in Kelvin, K] and
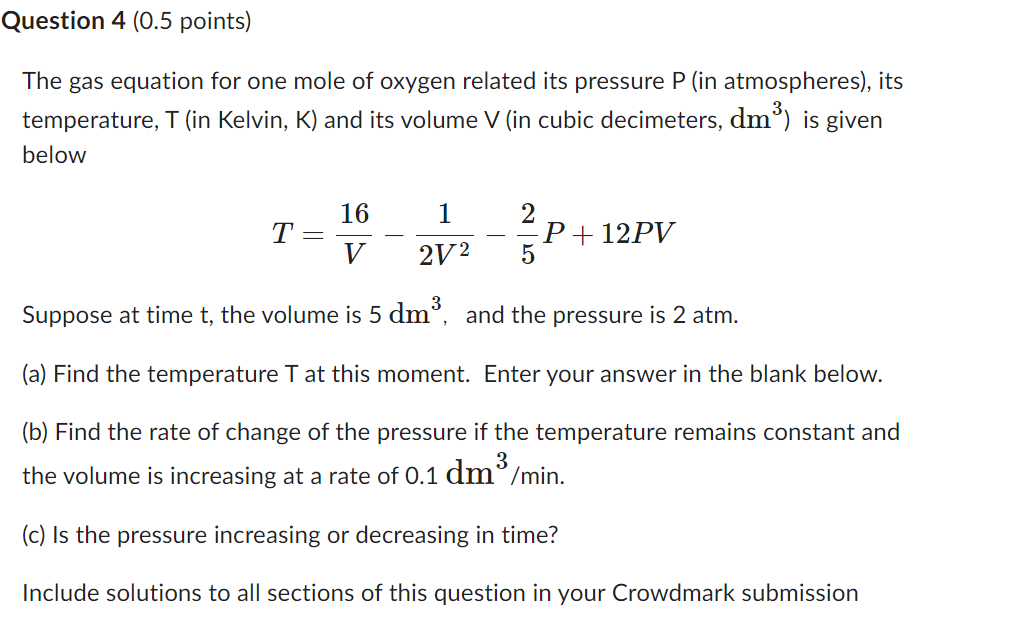
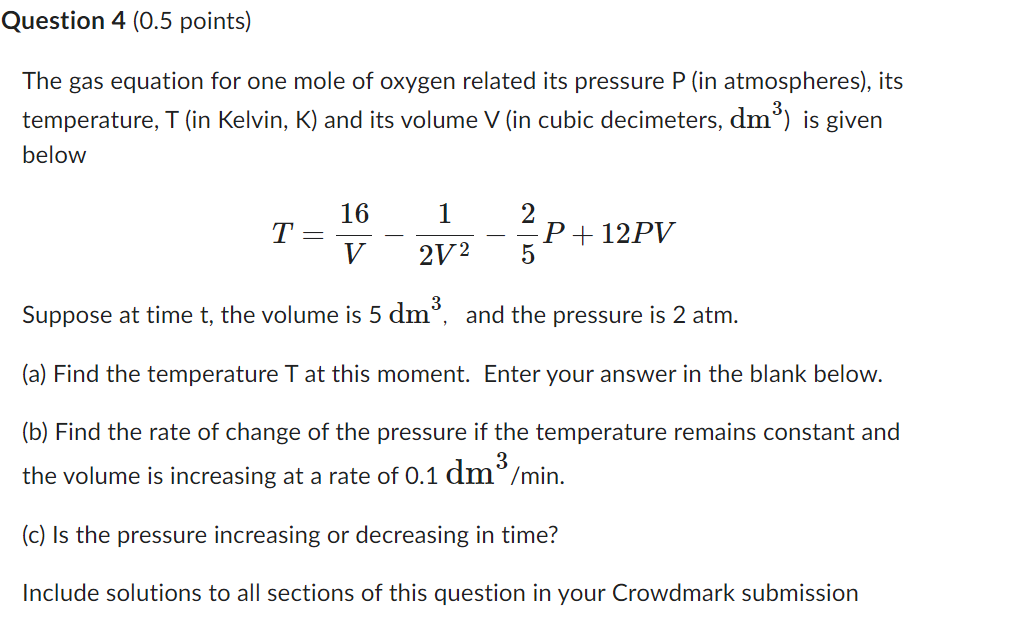
Question 4 (0.5 points) The gas equation for one mole of oxygen related its pressure P [in atmospheres), its temperature, T (in Kelvin, K] and its volume V (in cubic decimeters, dmgl is given below 16 1 2 T: P l 12PV V 2V2 5 Suppose at time t, the volume is 5 (11113. and the pressure is 2 atm. (a) Find the temperature T at this moment. Enter your answer in the blank below. (b) Find the rate of change of the pressure if the temperature remains constant and the volume is increasing at a rate of 0.1 de/min. (c) Is the pressure increasing or decreasing in time? Include solutions to all sections of this question in your Crowdmark submission
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


