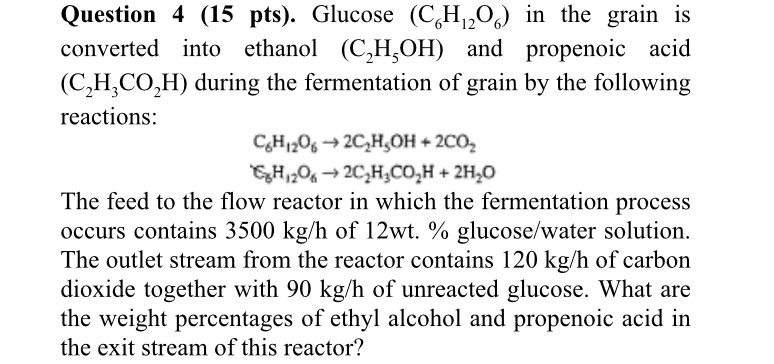
Question: Question 4 ( 1 5 pts ) . Glucose ( C 6 H 1 2 O 6 ) in the grain is converted into ethanol
Question pts Glucose in the grain is converted into ethanol and propenoic acid during the fermentation of grain by the following reactions:
The feed to the flow reactor in which the fermentation process occurs contains of glucosewater solution. The outlet stream from the reactor contains of carbon dioxide together with of unreacted glucose. What are the weight percentages of ethyl alcohol and propenoic acid in the exit stream of this reactor?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


