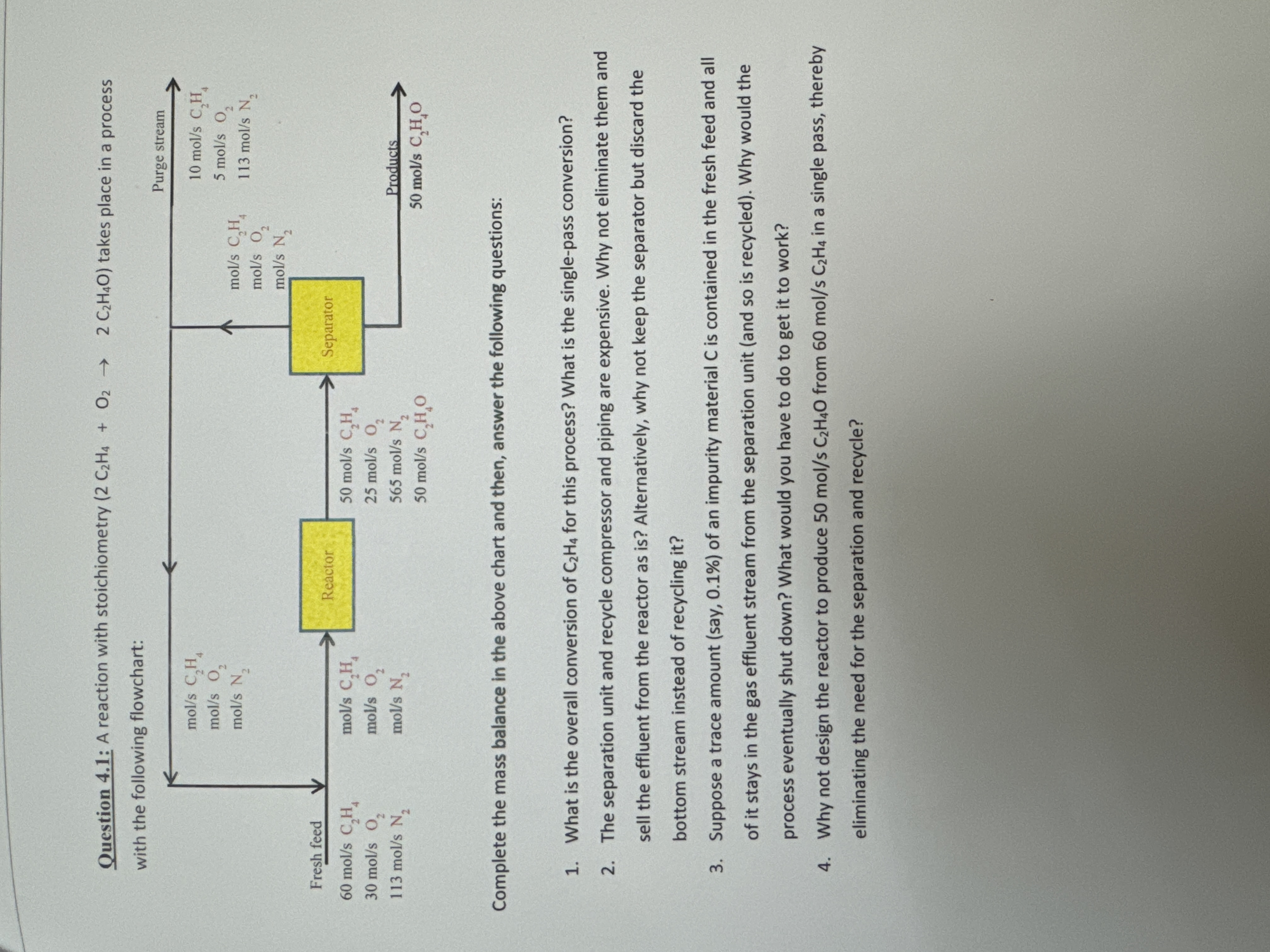
Question: Question 4 . 1 : A reaction with stoichiometry ( 2 C 2 H 4 + O 2 2 C 2 H 4 O )
Question : A reaction with stoichiometry takes place in a process
Complete the mass balance in the above chart and then, answer the following questions:
What is the overall conversion of for this process? What is the singlepass conversion?
The separation unit and recycle compressor and piping are expensive. Why not eliminate them and sell the effluent from the reactor as is Alternatively, why not keep the separator but discard the bottom stream instead of recycling it
Suppose a trace amount say of an impurity material is contained in the fresh feed and all of it stays in the gas effluent stream from the separation unit and so is recycled Why would the process eventually shut down? What would you have to do to get it to work?
Why not design the reactor to produce from in a single pass, thereby eliminating the need for the separation and recycle?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


