Question: Question 4 2 pts The average distance between collisions in a gas has doubled. If the process was isothermal, the pressure of the gas has
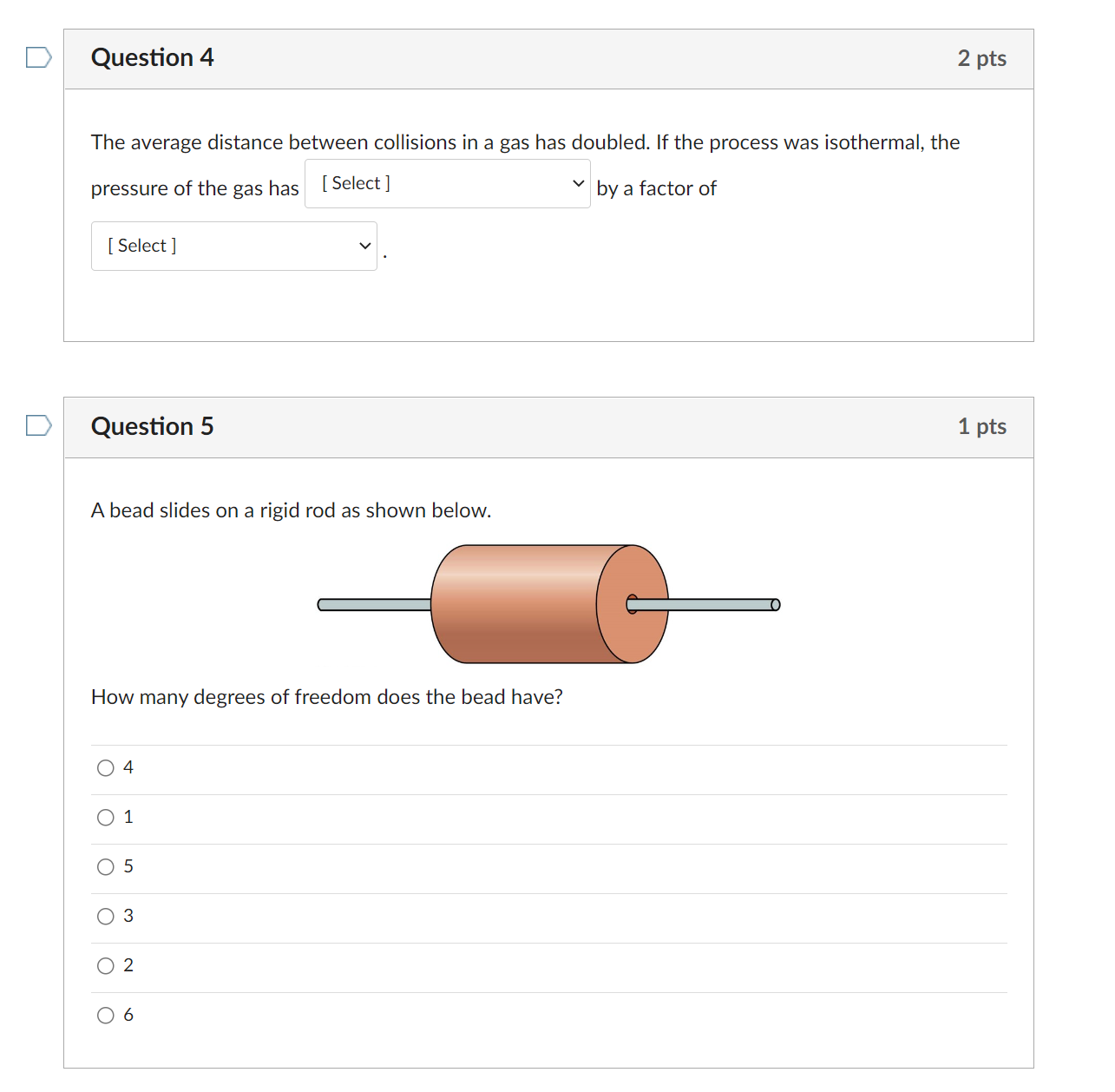
Question 4 2 pts The average distance between collisions in a gas has doubled. If the process was isothermal, the pressure of the gas has [59'9\"] V by a factor of [ Select ] v . Question 5 1 pts A bead slides on a rigid rod as shown below. How many degrees of freedom does the bead have? O4 Q1 05 03 o2 06 Question 6 1 pts An ice cube at 0C is placed in a very large bathtub lled with water at 30C and allowed to melt, causing no appreciable change in the temperature of the bath water. The system is the ice plus water. Which one of the following statements is true? O The entropy of the system increases because the process is irreversible. O The entropy of the water does not change because its temperature did not change. O The entropy gained by the ice cube is equal to the entropy lost by the water. O The entropy lost by the ice cube is equal to the entropy gained by the water. 0 The net entropy change of the system is zero because no heat was added to the system
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


