Question: Question 4: 22 marks (a) Determine the phase compositions for the methyl acetate (1)-cyclohexane (2) binary system at 64C and 68kPa, assuming Raoult's law is
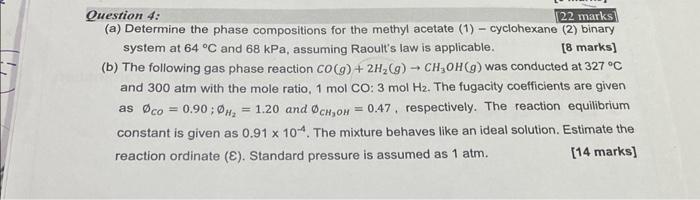
Question 4: 22 marks (a) Determine the phase compositions for the methyl acetate (1)-cyclohexane (2) binary system at 64C and 68kPa, assuming Raoult's law is applicable. [ 8 marks] (b) The following gas phase reaction CO(g)+2H2(g)CH3OH(g) was conducted at 327C and 300atm with the mole ratio, 1molCO:3molHH2. The fugacity coefficients are given as CO=0.90;H2=1.20 and CH3OH=0.47, respectively. The reaction equilibrium constant is given as 0.91104. The mixture behaves like an ideal solution. Estimate the reaction ordinate (). Standard pressure is assumed as 1atm. [14 marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


