Question: QUESTION 4 : Absorption and Stripping ( 2 0 ) Solvent C absorbs component A from a gaseous mixture of A and B into solvent
QUESTION : Absorption and Stripping
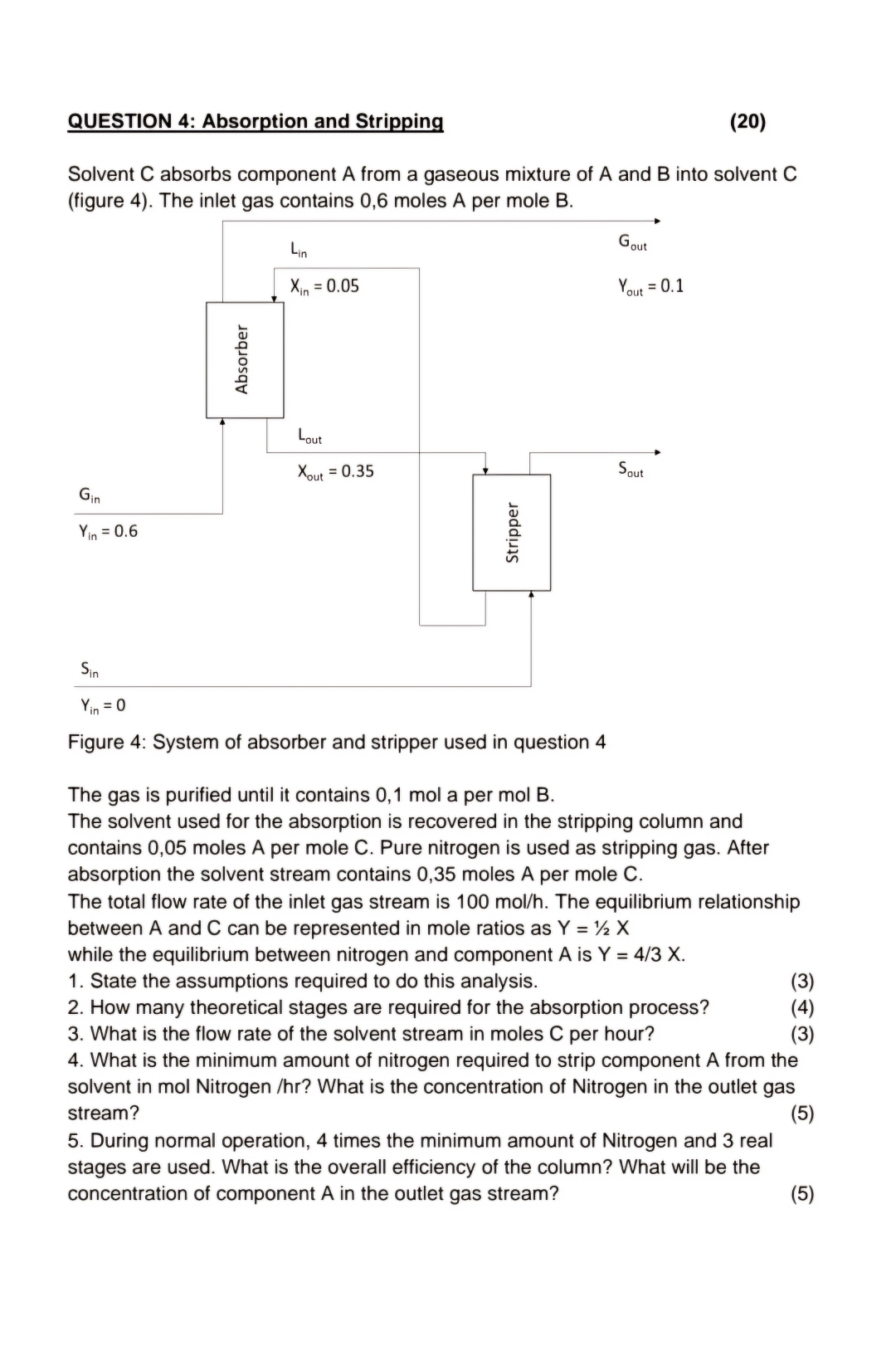
Solvent absorbs component A from a gaseous mixture of A and into solvent fiqure The inlet qas contains moles A per mole B
Figure : System of absorber and stripper used in question
The gas is purified until it contains mol a per mol B
The solvent used for the absorption is recovered in the stripping column and contains moles A per mole C Pure nitrogen is used as stripping gas. After absorption the solvent stream contains moles A per mole
The total flow rate of the inlet gas stream is The equilibrium relationship between A and can be represented in mole ratios as while the equilibrium between nitrogen and component is
State the assumptions required to do this analysis.
How many theoretical stages are required for the absorption process?
What is the flow rate of the solvent stream in moles per hour?
What is the minimum amount of nitrogen required to strip component A from the solvent in mol Nitrogen What is the concentration of Nitrogen in the outlet gas stream?
During normal operation, times the minimum amount of Nitrogen and real stages are used. What is the overall efficiency of the column? What will be the concentration of component in the outlet gas stream?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


