Question: QUESTION 4 Calculate the average atomic mass of a sample of sulfur has that has the following isotopes with the masses and relative abundances
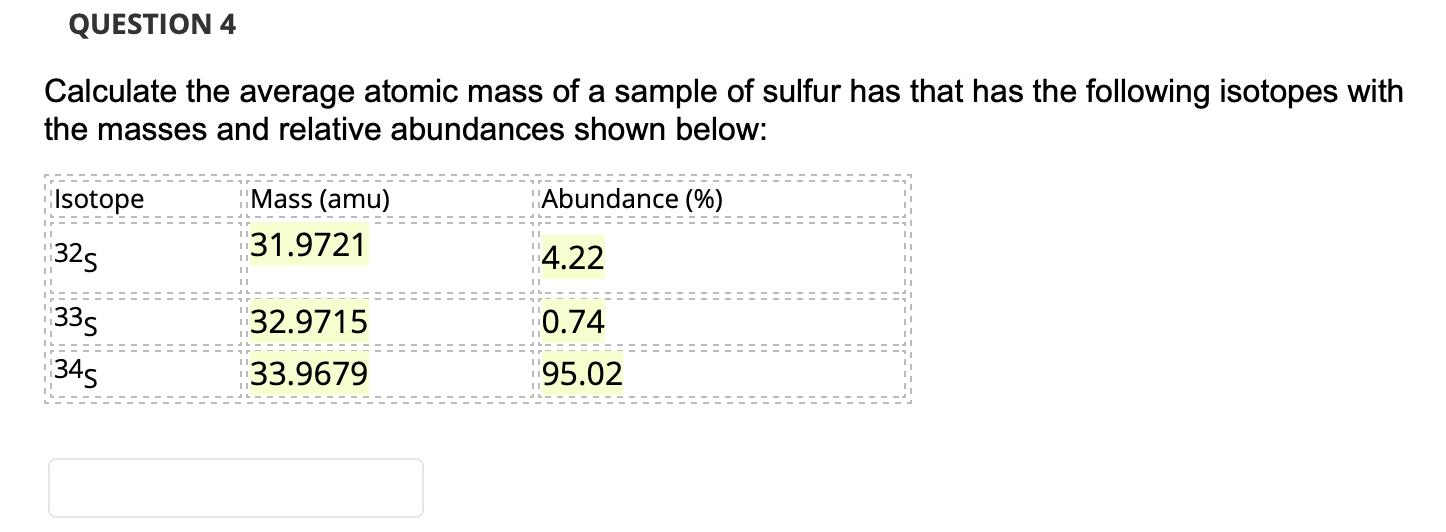
QUESTION 4 Calculate the average atomic mass of a sample of sulfur has that has the following isotopes with the masses and relative abundances shown below: Isotope Mass (amu) Abundance (%) 31.9721 325 4.22 335 32.9715 0.74 345 33.9679 95.02
Step by Step Solution
3.47 Rating (170 Votes )
There are 3 Steps involved in it
Solution The average atomic mass of a chemical element is ... View full answer

Get step-by-step solutions from verified subject matter experts


