Question: Question 4 How much work is done when 67g of C6H6 react in a combustion reaction at 313K ? 2C6H6(l)+15O2(g)12CO2(g)+6H2O(aq)H=6843
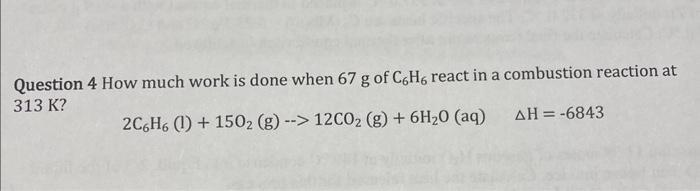
Question 4 How much work is done when 67g of C6H6 react in a combustion reaction at 313K ? 2C6H6(l)+15O2(g)12CO2(g)+6H2O(aq)H=6843
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


