Question: QUESTION 4 The binary VLE system C C l 4 ( 1 ) + EtOH ( 2 ) [ carbon tetrachloride - ethanol ] forms
QUESTION
The binary VLE system EtOH carbon tetrachloride ethanol forms an azeotrope at
kPa, At this temperature the vapour pressure of the two components are
kPa and kPa.
Assuming that the system obeys the Van Laar equations, calculate the pressure at which a CCEtOH liquid
mixture containing a mole fraction of would boil at
State any assumptions needed to solve the problem with the given data.
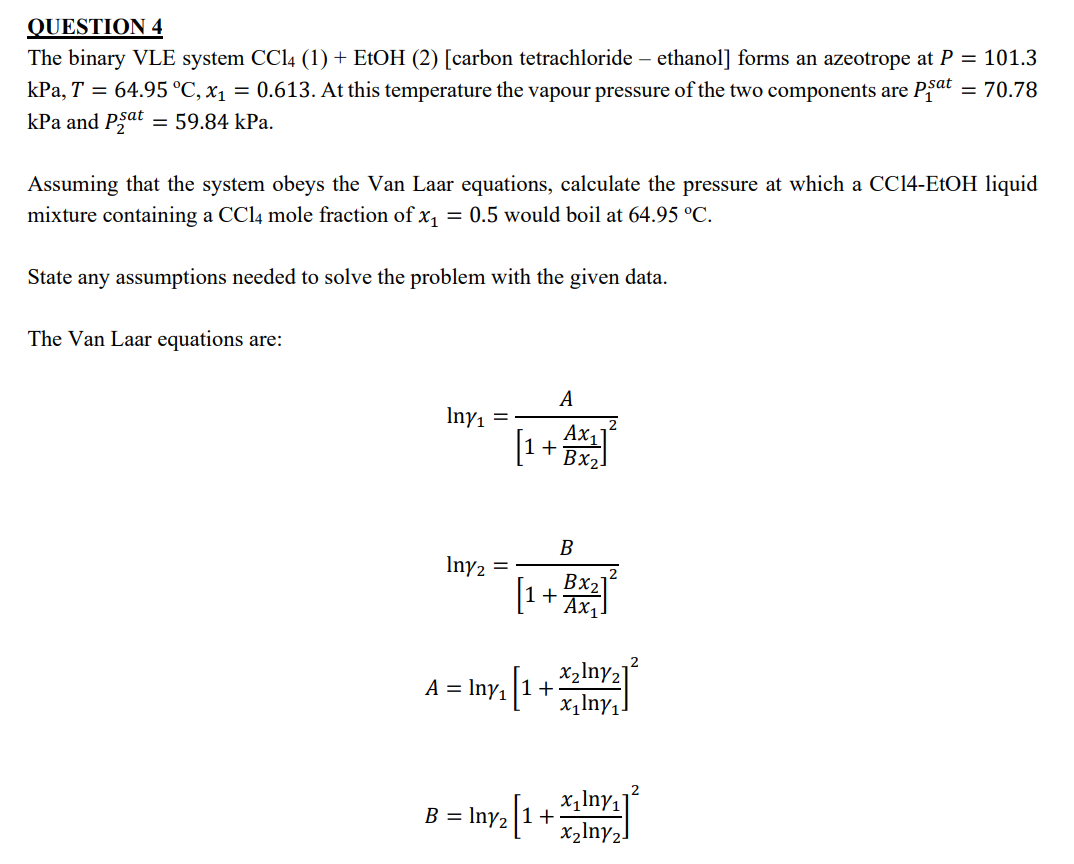
The Van Laar equations are:
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


