Question: Question 4 Type numbers in the boxes How many moles of electrons are produced by a current of 13.1 A running for 3.0 hour? (1

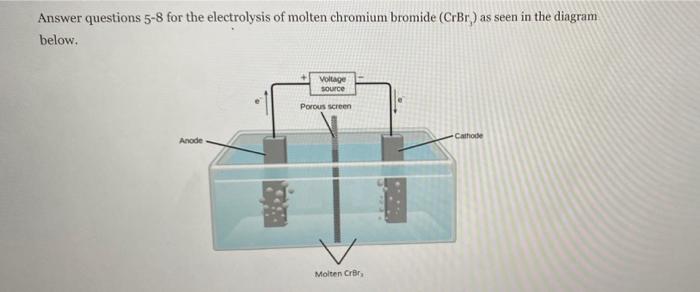
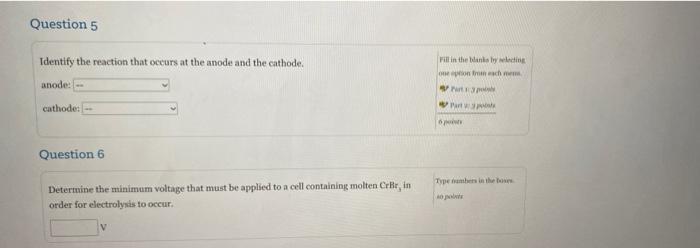
Question 4 Type numbers in the boxes How many moles of electrons are produced by a current of 13.1 A running for 3.0 hour? (1 faraday = 96,485 coulombs) 10 points mole Answer questions 5-8 for the electrolysis of molten chromium bromide (CrBr) as seen in the diagram below, Voltage Source Porous screen Anode Cathode Molten Cror Question 5 Identify the reaction that occurs at the anode and the cathode. Fill in the bally in onion rich Mart anode: cathodes Question 6 Types in the town Determine the minimum voltage that must be applied to a cell containing molten Crir in order for electrolysis to occur. V
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


