Question: Question 4: Using the conversion in question 3 finalise the MB at input-output level Question 5: calculate the volume of a PFR reactor ( in

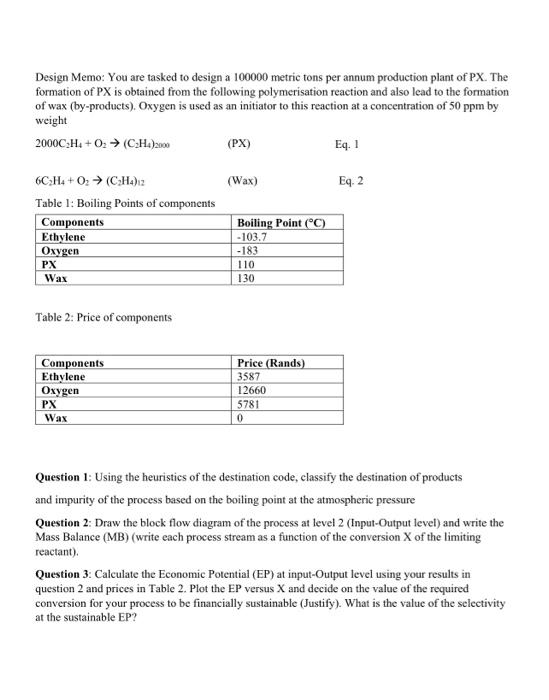
Question 4: Using the conversion in question 3 finalise the MB at input-output level Question 5: calculate the volume of a PFR reactor ( in m3 ) (no pressure drop, Isothermal reaction) to achieve the conversion obtained in Question 3 if the reaction rate for thermal initiation of ethylene is given by: rA=ktCA Eq. 3 Where the rate constant is, kt=8.21010exp[8.314T(84000+47P)] Eq. 4 Where, PTR=300MPa=500K(Averagetemperaturewhereoxygendecomposed)=8314MPadm3/mol.K Question 6: By plotting and looking at the Levenspiel Plot, do you think it was justified to use a PFR in question 5 (discuss)? Design Memo: You are tasked to design a 100000 metric tons per annum production plant of PX. The formation of PX is obtained from the following polymerisation reaction and also lead to the formation of wax (by-products). Oxygen is used as an initiator to this reaction at a concentration of 50ppm by weight 2000C2H4+O2(C2H4)2000 Eq. 1 6C2H4+O2(C2H4)12(Wax) Eq. 2 Table 1: Boiling Points of components Table 2: Price of components Question 1: Using the heuristics of the destination code, classify the destination of products and impurity of the process based on the boiling point at the atmospheric pressure Question 2: Draw the block flow diagram of the process at level 2 (Input-Output level) and write the Mass Balance (MB) (write each process stream as a function of the conversion X of the limiting reactant). Question 3: Calculate the Economic Potential (EP) at input-Output level using your results in question 2 and prices in Table 2. Plot the EP versus X and decide on the value of the required conversion for your process to be financially sustainable (Justify). What is the value of the selectivity at the sustainable EP
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


