Question: Question 5 (30 Marks) A mixture of 2 mol % butane (C4H10) and 98 mol% air is charged to a PFR under isothermal kmol and
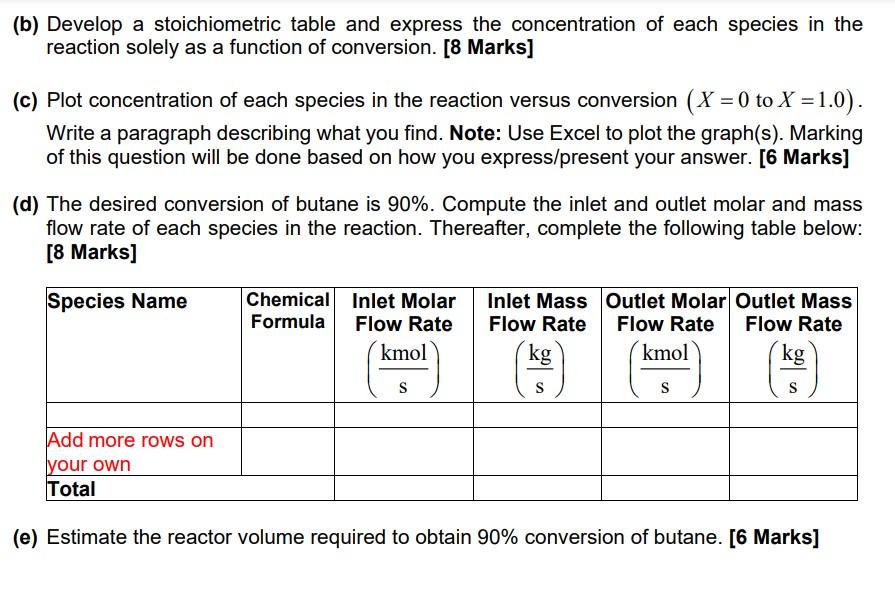
Question 5 (30 Marks) A mixture of 2 mol % butane (C4H10) and 98 mol% air is charged to a PFR under isothermal kmol and isobaric conditions, in which butane is oxidised to produce 0.0084 of maleic S anhydride (C4H2O3): CH0 +3.50 CHO3 + 4HO written symbolically as B+3.50 M + 4HO, in which B is butane (C4H0) and M is maleic anhydride (C4H2O3). The entering pressure is 220 kPa and the entering temperature is 703 K. All components are in the gas phase at the reactor temperature and pressure. The desired conversion of butane is 90%. It is assumed that the reaction is first-order in butane and the rate of formation of maleic 1 and C is the concentration -15,649 anhydride is r = k C where k=8.104810 exp B T S of butane in kmol m Note: T is in Kelvin. (a) Write the equations that relate the rate of consumption of butane and oxygen with the rate of formation of maleic anhydride and water. [2 Marks] (b) Develop a stoichiometric table and express the concentration of each species in the reaction solely as a function of conversion. [8 Marks] (c) Plot concentration of each species in the reaction versus conversion (X=0 to X = 1.0). Write a paragraph describing what you find. Note: Use Excel to plot the graph(s). Marking of this question will be done based on how you express/present your answer. [6 Marks] (d) The desired conversion of butane is 90%. Compute the inlet and outlet molar and mass flow rate of each species in the reaction. Thereafter, complete the following table below: [8 Marks] Species Name Chemical Formula Inlet Molar Inlet Mass Outlet Molar Outlet Mass Flow Rate Flow Rate Flow Rate Flow Rate kmol kg kmol kg S S S S Add more rows on your own Total (e) Estimate the reactor volume required to obtain 90% conversion of butane. [6 Marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


