Question: Question 5 1 pts Below is the balanced neutralization reaction for sodium hydroxide and sulfuric acid. If 90.0 mL of 1.50 M NaOH is neutralized
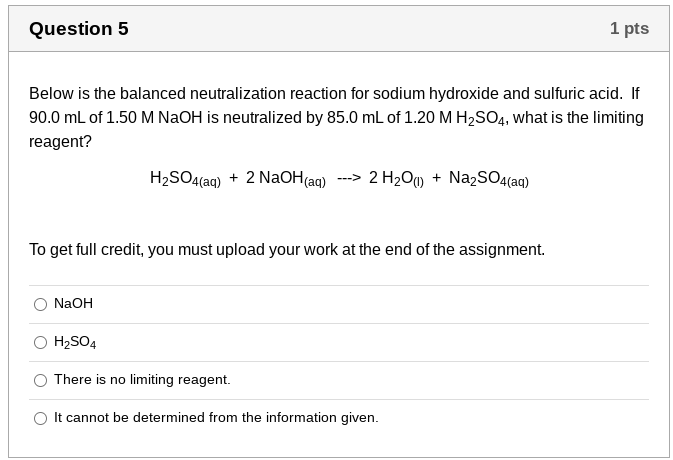
Question 5 1 pts Below is the balanced neutralization reaction for sodium hydroxide and sulfuric acid. If 90.0 mL of 1.50 M NaOH is neutralized by 85.0 mL of 1.20 M H2SO4, what is the limiting reagent? H2SO4(aq) + 2 NaOH(aq) ---> 2 H200) + Na2SO4(aq) To get full credit, you must upload your work at the end of the assignment. NaOH H2SO4 There is no limiting reagent. It cannot be determined from the information given
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


