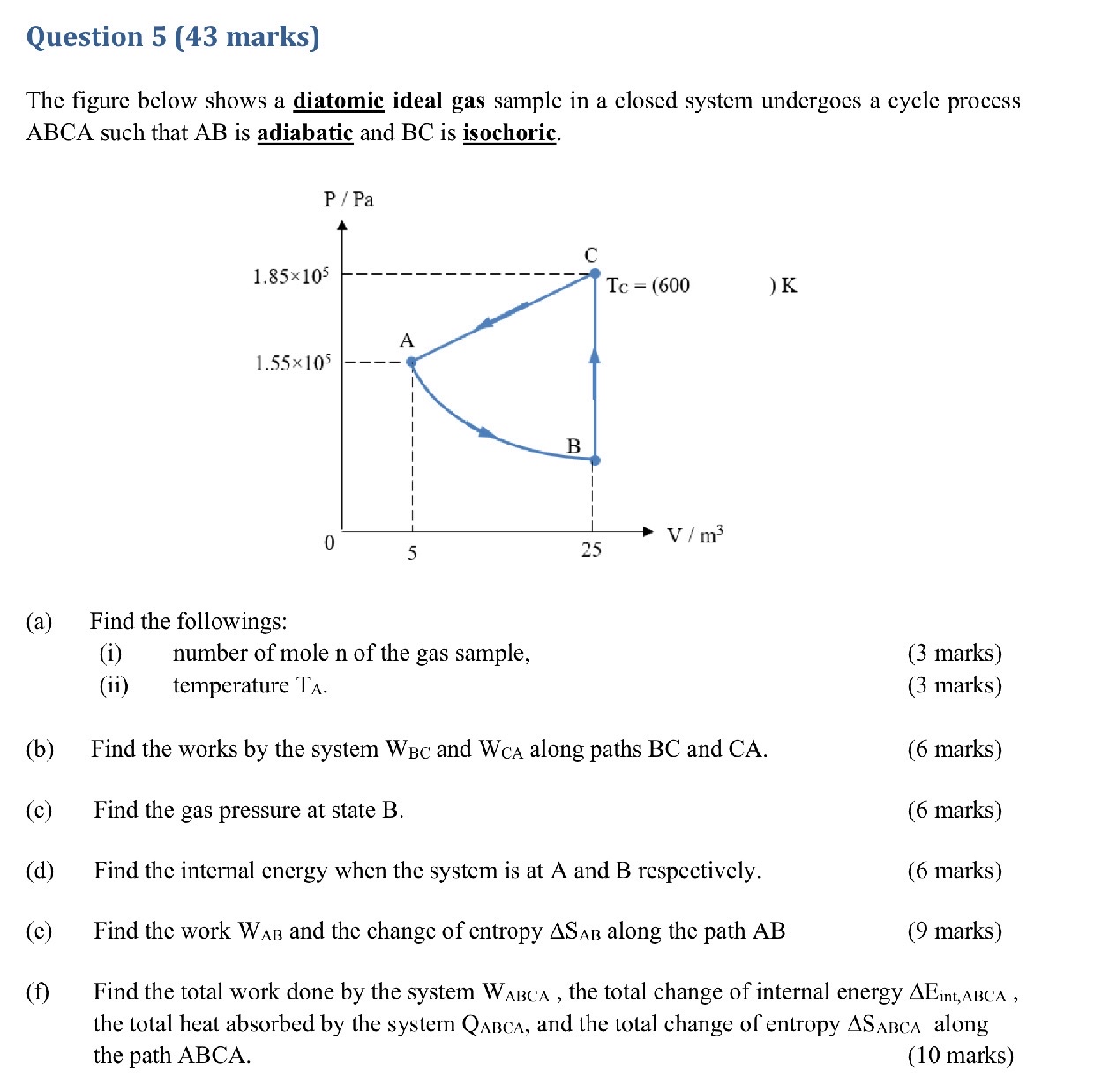
Question: Question 5 ( 4 3 marks ) The figure below shows a diatomic ideal gas sample in a closed system undergoes a cycle process ABCA
Question marks
The figure below shows a diatomic ideal gas sample in a closed system undergoes a cycle process ABCA such that is adiabatic and is isochoric.
K
a Find the followings:
i number of mole n of the gas sample,
marks
ii temperature
marks
b Find the works by the system and along paths BC and CA
marks
c Find the gas pressure at state B
marks
d Find the internal energy when the system is at A and B respectively.
marks
e Find the work and the change of entropy along the path
marks
f Find the total work done by the system the total change of internal energy the total heat absorbed by the system and the total change of entropy along the path ABCA
marks
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


