Question: Question 5 (4 points) The nitrous aciditrite buffer ( Ka = 4x10-4) has been measured to have a pH of 2.8. Calculate the ratio of

![ratio of [HNO2to (NO2) 1 [HNO2l / 4 [NO 21 12 [HNO2]](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f852ab57c4c_12266f852aae9741.jpg)
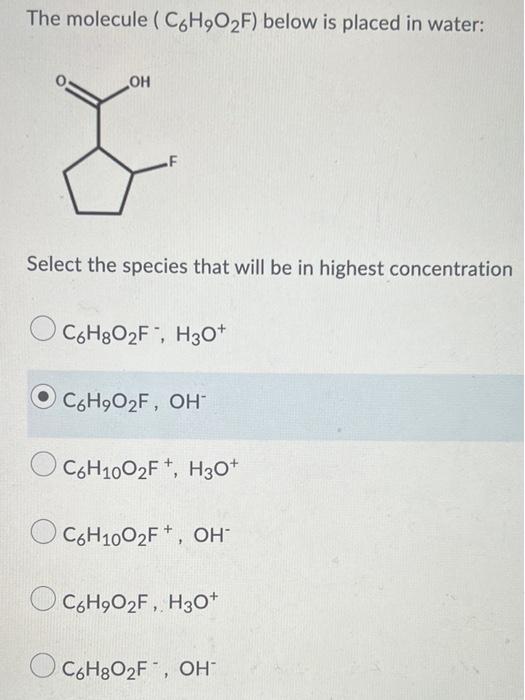
Question 5 (4 points) The nitrous aciditrite buffer ( Ka = 4x10-4) has been measured to have a pH of 2.8. Calculate the ratio of [HNO2to (NO2) 1 [HNO2l / 4 [NO 21 12 [HNO2] / 1 [NO2) 4 [HNO2l/1 [NO2] 1 [HNO2] / 12 [NO2] The molecule (C6H2O2F) below is placed in water: & F Select the species that will be in highest concentration C6H8O2F, H30+ O CoH,O2F, OH CoH1002F+, H30+ O C6H1002F+, OH O CoH902F, H30+ + O CoHgO2F", OH Question 12 (4 points) % A- vs pH 100 90 80 70 60
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


