Question: Question 5: Give one specific reason explaining your percent error in question 4 Question 4 is showed as well as all other questions and data.

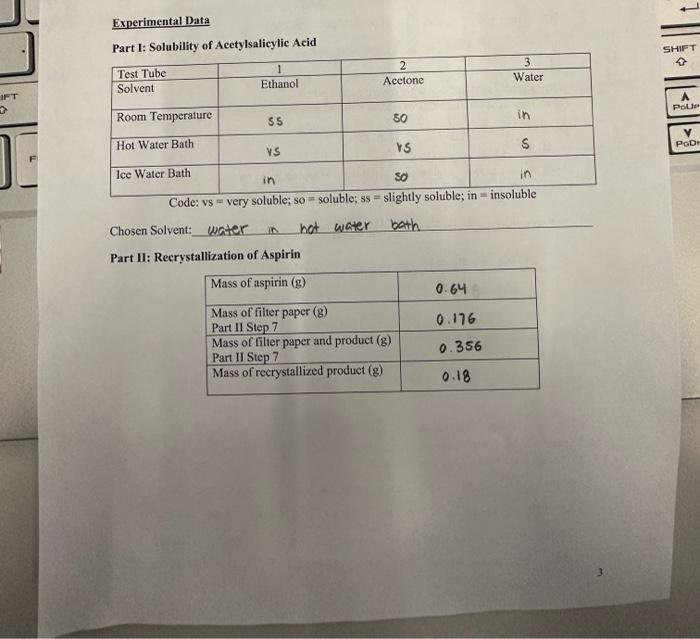
Data Analysis: Show your work 1. How many mg of aspirin did you recover from the crushed tablets (what was the mass of your recrystallized product)? 180mg of aspinin were recovered 2. How many mg of aspirin did you recover from each table?? 90mg of aspinng fram each tablet 2180=90 3. What pereent of the erushed tablets were pure aspirin? (Mass recovered/Initial Mass of tablets " 100% ) 0.64g0.18100%=28% 4. Compare your answer in question 2 to the mass of aspirin listed on the bottle. What is the percent error in your experimental data compared to the actual mass of aspirin in each tablet? PercentErtor=Measuredmass(frombottle)|Mcasuredmass(frombottle)-Massyouobtainedi100325mg325mg10mg100=72% 5. Give 1 specifie reason explaining your percent error in question 4. 6. Explain why you chose your reerystallization solvent. What properties did this solvent have that made it the best for recrystallization? we chose water as oor solvent os \# was the only solvent that was only soluble in the hot water bath Using acetone or ethanol would have caused the recrystanlization to fal before the last step in hot water. Part I: Solubility of Acetylsalicylic Acid Code: vs = very soluble; so = soluble; ss = sligntiy soiuote; in - usvuuve Chosen Solvent: water in nat water bath Part II: Reerystallization of Aspirin
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


