Question: Question 6 (3 points) Given the equilibrium reaction between DHAP and GAP as below: At pH 7 and 25C, the standard Gibbs free energy of
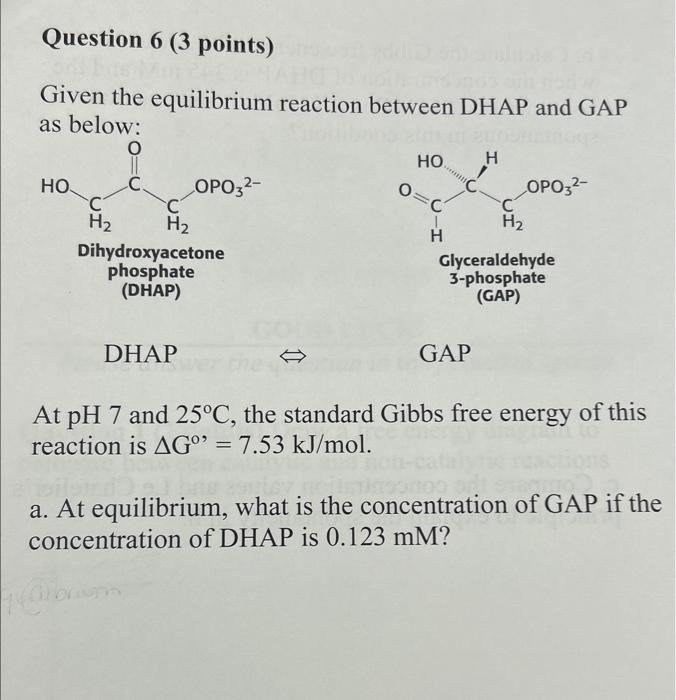
Question 6 (3 points) Given the equilibrium reaction between DHAP and GAP as below: At pH 7 and 25C, the standard Gibbs free energy of this reaction is G=7.53kJ/mol. a. At equilibrium, what is the concentration of GAP if the concentration of DHAP is 0.123mM ? b. Calculate the Gibbs free energy of the reaction G when the concentration of DHAP is 345mM and the concentration of GAP is 0.78mM. Is the reaction spontaneous in this condition? c. Compare the concentration values and Le Chatelier's principle to explain the spontaneity in b
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


