Question: Question 7 (1 point) 2. C + Consider the following reactions for formation of four different compounds: 1. C19+026) - COCO A,H' = -110.5 kJ/mol
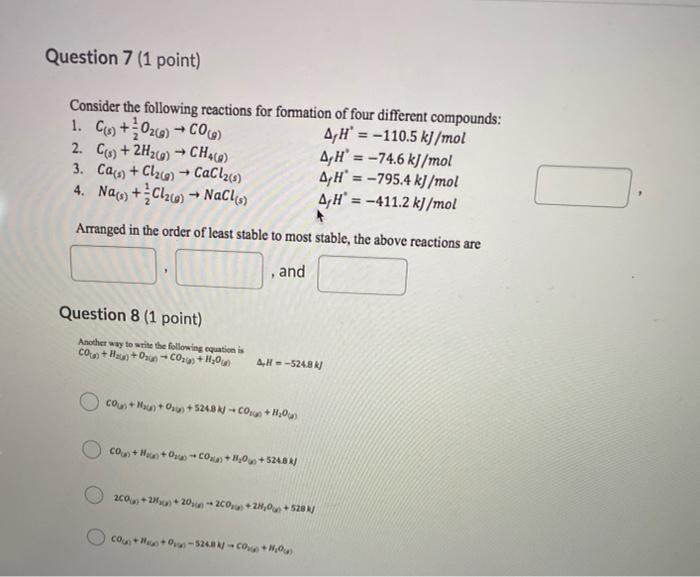
Question 7 (1 point) 2. C + Consider the following reactions for formation of four different compounds: 1. C19+026) - COCO A,H' = -110.5 kJ/mol -2H26) - CH4C) A, H = -74.6 kJ/mol 3. Ca(s) + Cl26) - CaCl2(8) A,H' = -795.4 kJ/mol 4. Naw+C126) -- NaClo) A, H = -411.2 kJ/mol Arranged in the order of least stable to most stable, the above reactions are and Question 8 (1 point) Another way to write the following equation is CO+H4+0xC020+H,000 AH = -5248 Cou) + x + 0,02+5248 N -C0169 + H206) COU+H+04-C0H,06 +5248R) 200+20+20 - 200, +2, +528 c. + V + 0,4 -51% = 60 + 100g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


