Question: Question 7 For solutions of acetone ( ac ) plus chloroform ( chl ) at 3 5 . 2 C , vapor pressures P and
Question
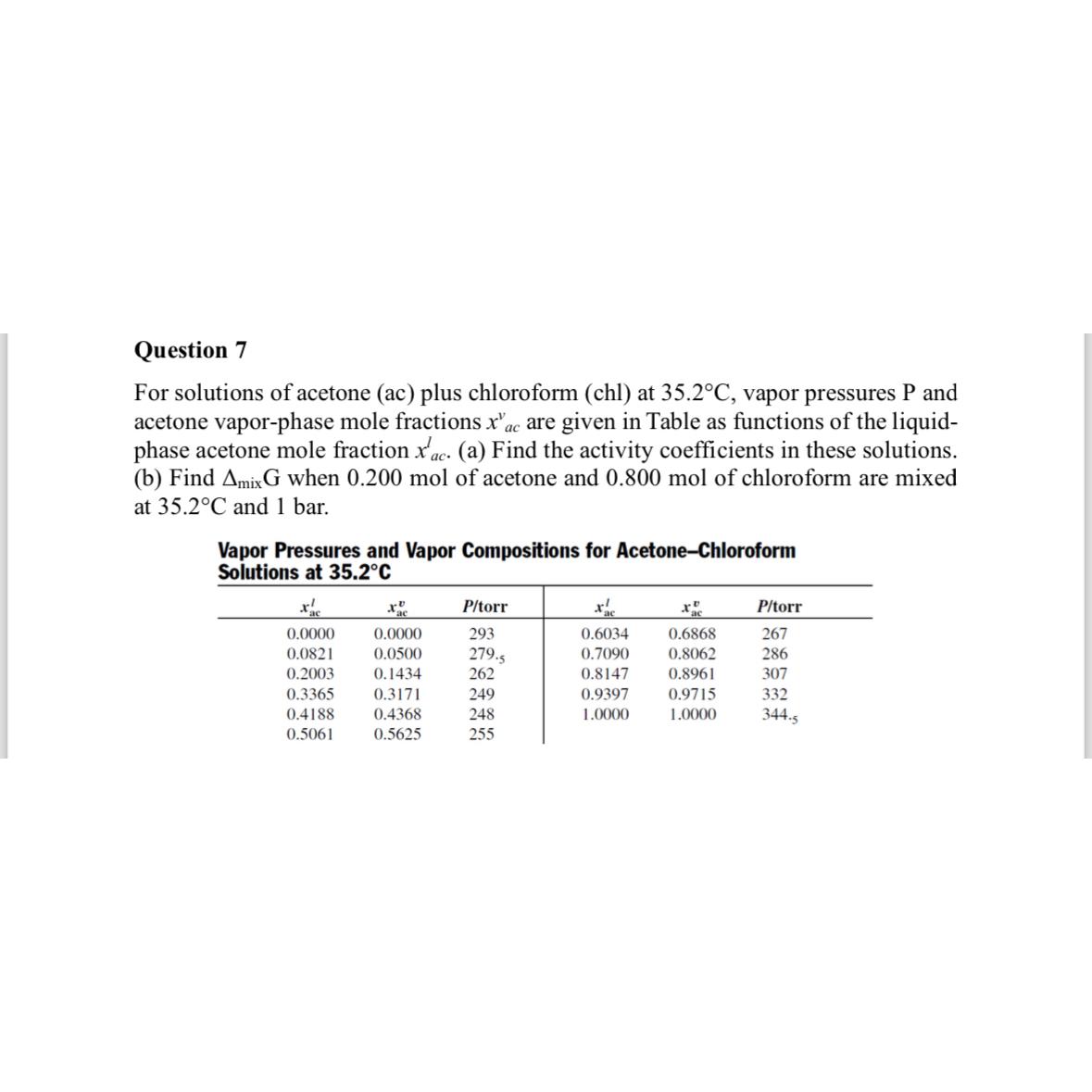
For solutions of acetone ac plus chloroform chl at vapor pressures and acetone vaporphase mole fractions are given in Table as functions of the liquidphase acetone mole fraction a Find the activity coefficients in these solutions. b Find when mol of acetone and mol of chloroform are mixed at and bar.
Vapor Pressures and Vapor Compositions for AcetoneChloroform Solutions at
table torr, torr
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


