Question: Question 8 (1 point) Which net charge calculation could be true for an amino acid in aqueous solution -carboxylate: 1; -amine: +1; sidechain: +1; net:
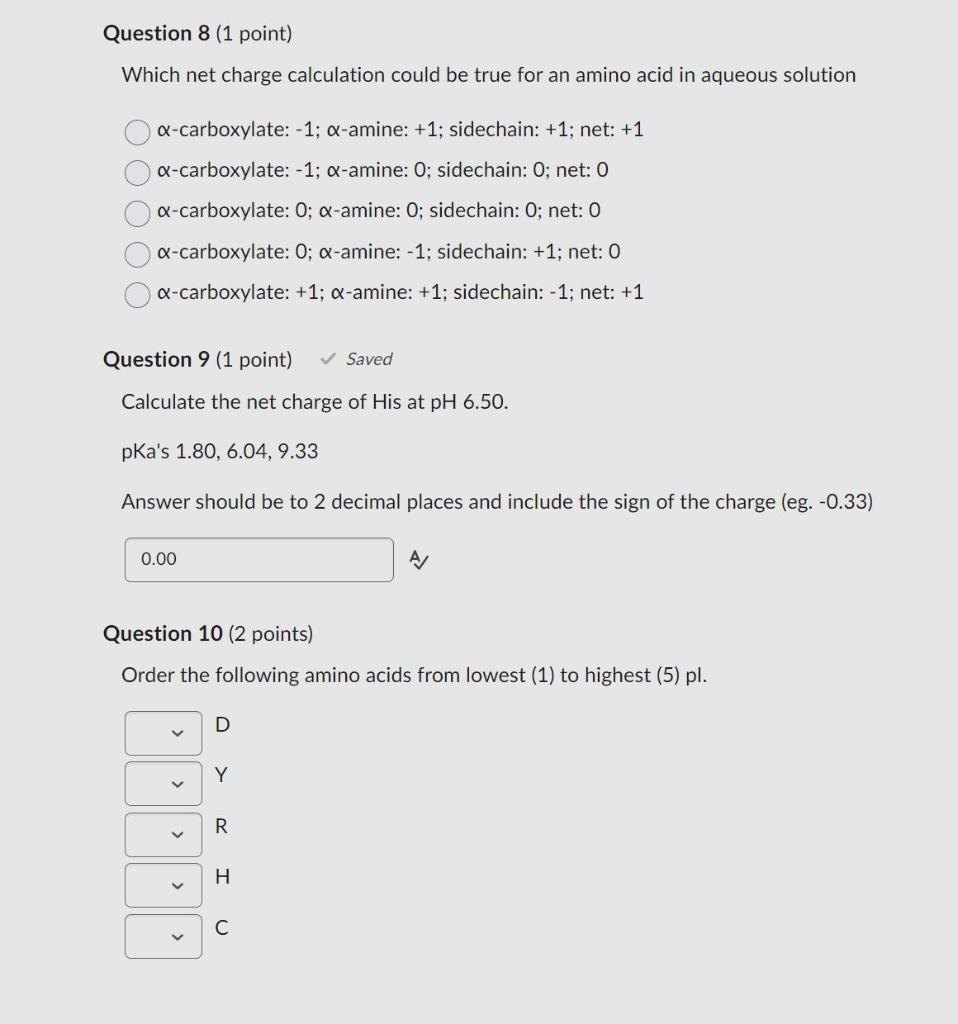
Question 8 (1 point) Which net charge calculation could be true for an amino acid in aqueous solution -carboxylate: 1; -amine: +1; sidechain: +1; net: +1 -carboxylate: -1; -amine: 0 ; sidechain: 0; net: 0 -carboxylate: 0; -amine: 0 ; sidechain: 0 ; net: 0 -carboxylate: 0;-amine: 1; sidechain: +1; net: 0 -carboxylate: +1;-amine: +1; sidechain: 1; net: +1 Question 9 (1 point) Saved Calculate the net charge of His at pH6.50. pKa's 1.80,6.04,9.33 Answer should be to 2 decimal places and include the sign of the charge (eg. -0.33) A Question 10 (2 points) Order the following amino acids from lowest (1) to highest (5) pl. D Y R H C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


