Question: Question 8 10 points For the reaction below, how many moles of phosphate rock (Cas(PO4)3F) is required to make 10 moles of calcium sulfate (Cas04)?

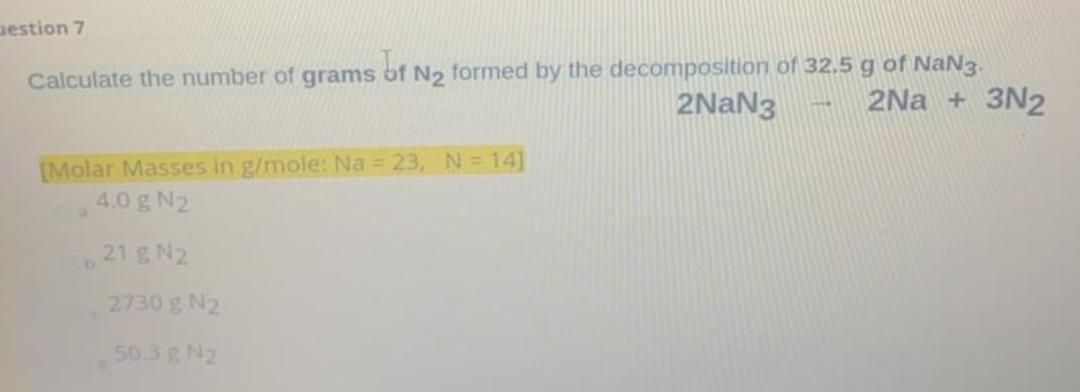



Question 8 10 points For the reaction below, how many moles of phosphate rock (Cas(PO4)3F) is required to make 10 moles of calcium sulfate (Cas04)? Ca5(PO4)3F+5H2504 --> 3H3PO4 + HF + 5CaSO4 10 moles of Cas(PO4)3F 0.1 moles of Cas(PO4)3F 2 moles of Cas(PO4)3F 50 moles of Cag(PO4)3F estion 7 Calculate the number of grams of Ny formed by the decomposition of 32,5 g of NaN3. 2NaN3 2Na + 3N2 [Molar Masses in g/mole: Na = 23, N = 14] 4.0 g N2 o 21 g N2 2730 g N2 50.3 g N2 Question 6 For the reaction C3H8 +502 + 3C02 + 4H20 The number of grams of CO2 that can be produced from 2.0 moles of C3Hg is [Molar Masses in g/mole: C = 12, H = 1, 0 = 16] a 264 g b. 100 g Cc 88g a 44 g For the reaction N2 + 3H2 2NH3 The number of moles of N2 required to react with 3.5 moles of H2 is [Molar Masses in g/mole: N = 14, H = 1] 3.6 moles N2 1.2 moles N2 Ob 10.8 moles N2 . 0.83 moles N2 d. Question 4 10 poir For the reaction below of phosphate rock (Ca5(PO4)3F) with sulfuric acid (H2SO4), the mole ratio of sulfuric acid to phosphate rock is Cas(PO4)3F+5H2504 --> 3H3PO4 + HF + 5CaSO4 3 moles O a. 1 moles 1 moles Ob. 5 moles O moles Oc 5 moles 5 moles Od. 1 moles
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


